Figure 1. Triadin200–232 and triadin200–231 peptides bind to RyR1 and exhibit a disordered secondary structure.
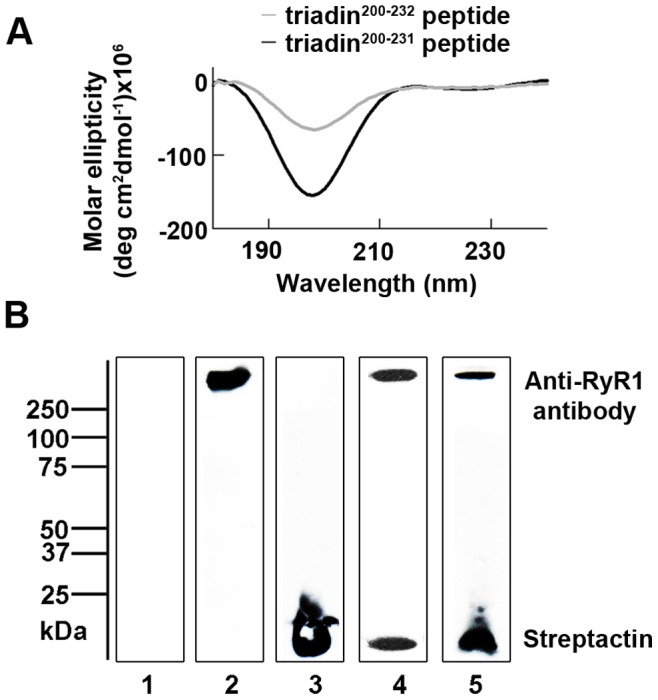
(A) Circular dichroism spectra averaged from four scans and corrected for the 10 mM sodium phosphate buffer are shown. Both triadin peptides (triadin200–231 (black trace) and triadin200–232 (grey trace); 0.03 mg/ml) show a negative peak at ∼197 nm, consistent with an intrinsically disordered structure. A positive peak at ∼190 nm and negative peaks at ∼208 and ∼223 nm (indicative of α-helical secondary structure) or a positive peak at ∼195 nm and a negative peak at ∼217 nm (indicative of β-sheet secondary structure), are absent. (B) Western blot, following streptavidin-agarose affinity chromatography, showing the association of RyR1 with biotin tagged triadin peptide. The upper half of the membrane was probed with anti-RyR1 antibody and the lower half was probed with Streptactin-HRP conjugate to identify the biotin tagged peptides. Lane 1 protein sample eluted from streptavidin-agarose incubated with RyR1 alone; Lane 2 purified RyR1 alone (control); Lane 3 biotin tagged triadin200–231 peptide alone (control); Lanes 4 and 5 protein sample eluted from streptavidin-agarose affinity chromatography, where streptavidin-agarose was incubated with biotin tagged triadin200–232 or triadin200–231 peptide respectively, prior to incubation with RyR1.
