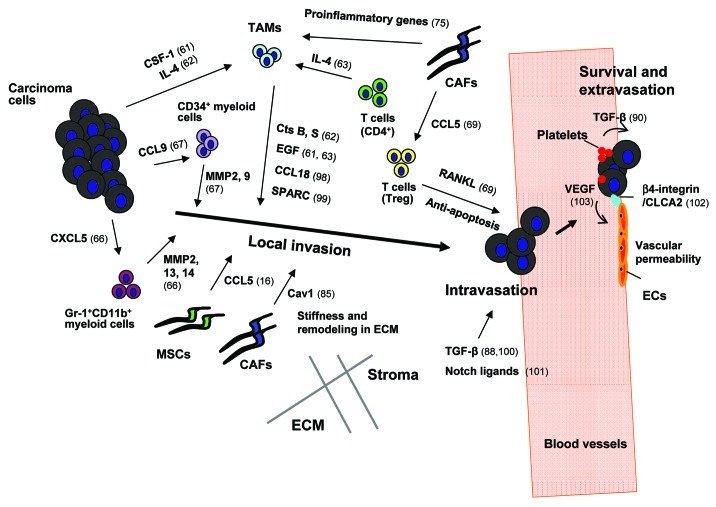
Figure 1. The roles of tumor-associated stroma in the induction of local invasion, intravasation, survival and extravasation of carcinoma cells. Breast cancer cells secrete colony-stimulating factor-1 (CSF-1), a hematopoietic growth factor recruiting tumor-associated macrophages (TAMs) into the primary tumor.61 Infiltrating perivascular TAMs secrete epidermal growth factor (EGF) which chemotactically attracts cancer cells toward the vasculature and thereby facilitates their intravasation.61 In addition, IL4 produced by breast cancer cells and CD4+ T lymphocytes boosts expression of EGF and cathepsin (Cts) B and S proteases by TAMs and further promotes TAM-instigated cancer cell invasion.62,63 In addition to the aforementioned factors TAM-derived CCL18 has also been shown to promote invasion of breast carcinoma cells via signaling through its cognate receptor, PITPNM3,98 whereas TAM-secreted protein acidic and rich in cysteine (SPARC) induces cancer cell migration by acting through αvβ5 integrin.99 CCR1+CD34+ immature myeloid cells respond to CCL9 chemokine secreted from colorectal carcinoma cells, and infiltrate the invasive front of the tumor epithelium, where they produce metalloproteinases (MMPs) 2 and 9.67 CXCL5, another chemokine produced by breast cancer cells, attracts a different population of immunosuppressive myeloid cells (Gr-1+CD11b+) toward the invasive front of tumor tissues where they produce MMP2, 13 and 14 facilitating invasion and metastasis of cancer cells.66 Similarly, CCL5 chemokine secreted by mesenchymal stem cells (MSCs) enhances invasion and metastasis of breast carcinoma cells by activating the CCR5 receptors on these cells.16 Caveolin-1 (Cav1) expressed on carcinoma-associated fibroblasts (CAFs) facilitates tumor invasion through the force-dependent architectural regulation of extracellular matrix (ECM), including its stiffening.85 The primary tumor microenvironment is a source of abundant TGF-β which induces transient activation of TGF-β signaling in breast carcinoma cells promoting their motility and intravasation.88 In addition, stromal-derived TGF-β stimulates expression of angiopoietin-like 4 (ANGPTL4) by breast cancer cells; ANGPTL4 primes cancer cells to dissociate cell-cell junctions between vascular endothelial cells and thereby increases the levels of their extravasation.100 Notch ligands, DLL4 and/or Jagged1 (Jag1), expressed by tumor-associated endothelial cells (EC), macrophages and fibroblasts have been shown to mediate Notch signaling-dependent invasion of colon cancer cells.101 Activation of NFκB signaling in CAFs mediated by IL-1β secreted from infiltrating immune cells results in the production of pro-inflammatory chemokines (e.g., CXCL1 and CXCL2) which chemotactically recruit TAMs in the primary tumor,75 whereas CCL5 secreted by CAFs signals via the CCR1 receptor expressed on regulatory T cells (T-reg).69 The recruited T-reg can express RANKL, a ligand for the RANK receptor on the surfaces of breast cancer cells.69 Signaling mediated upon activation of RANK resulting in IKKα and thus NFκB activation allows cancer cells to evade apoptosis and facilitates their extravasation. Platelet-derived TGF-β induces the TGF-β-Smad2/3 signaling in cancer cells, whereas direct physical contact between platelets and tumor cells results in activation of the NFκB pathway.90 Activation of both forms of signaling leads to induction of the EMT phenotype in CTCs that increases their extravasation.90 hCLCA2, a Ca2+-sensitive chloride channel protein which is expressed by ECs, is implicated in directing β4-integrin-dependent adhesion between these cells and breast carcinoma cells, allowing the latter to undergo extravasation effectively.102 Vascular endothelial growth factor (VEGF) secreted by tumor cells also aids tumor cell extravasation via induction of Src signaling in ECs and the resulting disruption of the integrity of the EC barrier/layer.103

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.