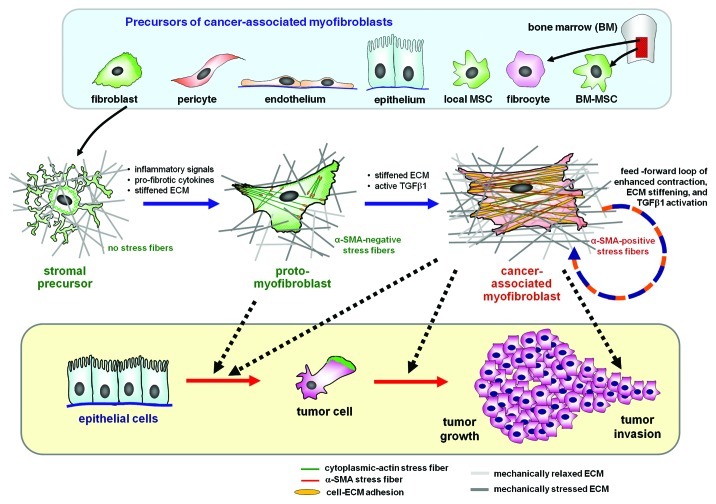
Figure 1. The myofibroblast in the tumor stroma. Myofibroblasts associated with the tumor stroma can be activated from a variety of different progenitor cells, including locally residing fibroblasts, epithelial and endothelial cells via epithelial-to-mesenchymal transition, pericytes and bone marrow-derived circulating fibrocytes and mesenchymal stem cells (MSCs). In the intact tissue, the precursor cells are stress-shielded by a functional extracellular matrix (ECM); they do not develop contractile features and cell-matrix adhesions. Upon injury and loss of tissue homeostasis inflammatory signals activate stromal cells to spread and remodel the initially soft ECM. The gradual increase in ECM stiffness permits the formation of contractile microfilament bundles of cytoplasmic actins (stress fibers) that characterize the proto-myofibroblast. Transforming growth factor-β1 (TGFβ1) in conjunction with the stiff ECM stimulates proto-myofibroblasts to express and incorporate α-smooth muscle actin (α-SMA) into stress fibers. The force generated by α-SMA-containing stress fibers leads to further ECM contraction, thereby establishing a mechanical feedback loop. The chemical and mechanical environment created by proto-myofibroblasts and differentiated myofibroblasts support epithelial cell transformation, invasion and tumor growth.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.