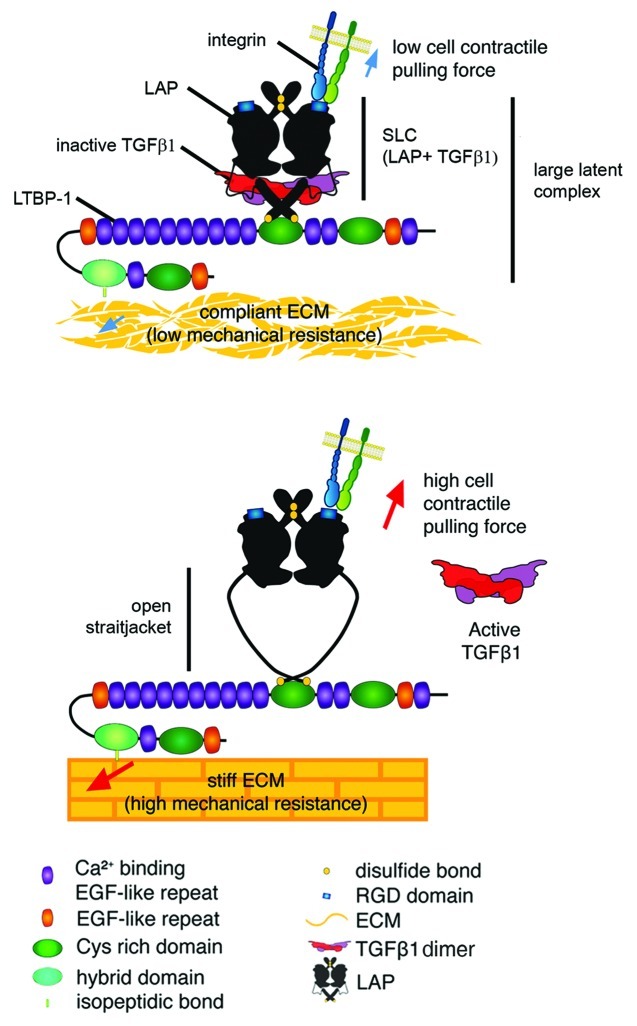
Figure 2. Integrin-mediated TGFβ1 activation by cell contraction. Structural and force spectroscopy data suggest that the transforming growth factor-β1 (TGFβ1) and latent TGFβ1 binding protein-1 (LTBP-1) binding domains of the latency-associated peptide (LAP) act as a sensor in a mechanical model of integrin-mediated TGFβ1 activation. Mechanical stretch, applied through cell integrins will open the latent TGFβ1 complex to release TGFβ1. Flexible domains in the LAP that are prone to unfolding lie within a domain that has been coined “straitjacket.”179 In the context of a poorly organized and compliant matrix and when cells develop only low contractile activity, the lack of sufficient mechanical tension will prevent the integrin-mediated conformational changes that are required to activate TGFβ1 from the latent complex. Conversely, on a stiff matrix the transmission of contractile forces via integrins to the LAP will favor unfolding of the straitjacket region, resulting in TGFβ1 release. Reproduced with permission.184

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.