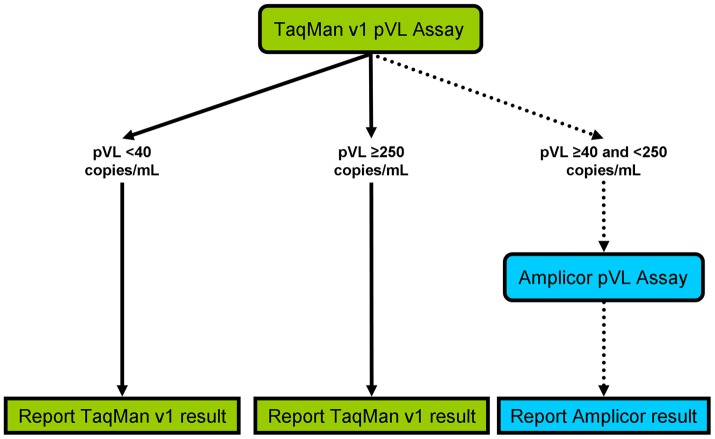
Figure 1. Plasma viral load testing and reporting protocol in British Columbia between October 2009 and April 2010.
All plasma samples were initially tested with the Roche COBAS AmpliPrep/COBAS TaqMan v1 HIV-1 Test (“TaqMan v1”) assay. TaqMan v1 pVL results <40 or ≥250 copies/mL were reported to physicians. Samples with TaqMan v1 pVL ≥40 and <250 copies/mL were re-tested by the Roche COBAS AmpliPrep/COBAS AMPLICOR HIV-1 MONITOR UltraSensitive Test, version 1.5 (“Amplicor v1.5”). The Amplicor v1.5 test results were reported to physicians.