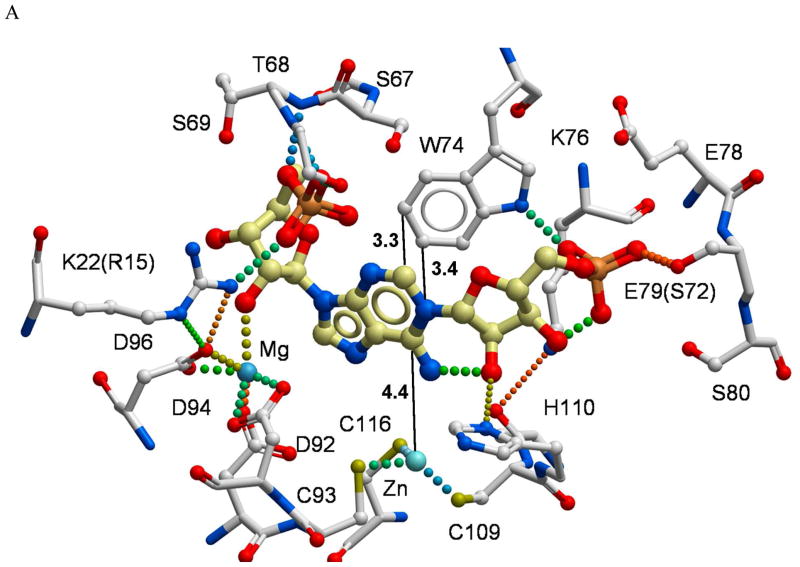
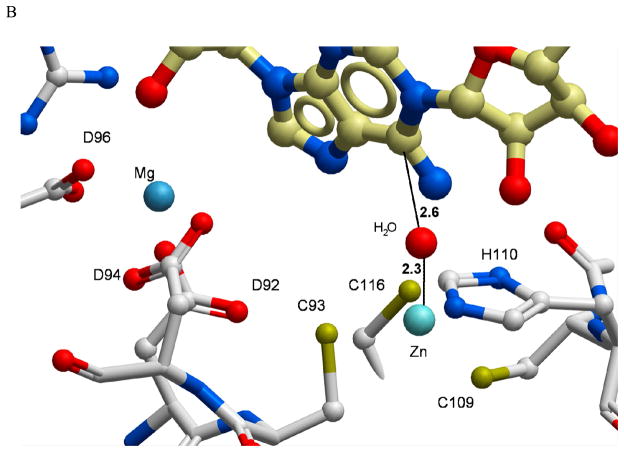
Figure 6.
A structural model of the PR-AMP cyclohydrolase active site with substrate docked. (A) The metal Zn2+ and Mg2+ binding sites and their relationships to the conserved amino acid residues are indicated to assess the functional roles in substrate binding site. The 16 residues labeled in the figure are among the most highly conserved residues described in Figure 1 and reside within 4 Å of the substrate binding site. (B) To better understand the potential positioning of the active site for a zinc-activated water attack on the C6 of the purine ring, a molecule of water was manually positioned into the substrate-docked model. Distances (black dotted lines) are shown in angstroms (Å). Hydrogen bonds and coordinative bonds to metals are rendered as colored spheres.