Figure 1.
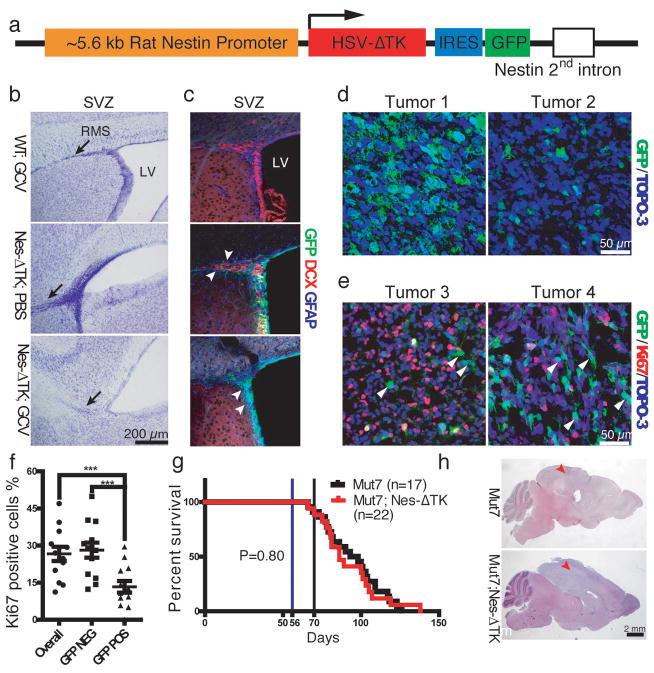
Characterization of the Nes-ΔTK-GFP transgene. a, Diagram of the Nes-ΔTK-GFP transgene. b,c, GCV administration ablates neural stem cells (NSCs) in wild type mice. b, Representative Nissl staining of the subventricular zone (SVZ) region in wild type mice treated with GCV (WT;GCV), Nes-ΔTK transgene mice treated with PBS (Nes-ΔTK;PBS), and Nes-ΔTK transgene mice treated with GCV (Nes-ΔTK;GCV); black arrows indicate the stem cell rostral migratory stream (RMS), which is greatly reduced in the Nes-ΔTK;GCV mice. c, GFP (transgene), GFAP (quiescent neural stem cells) and DCX (committed neural progenitors) immunostaining of the subventricular zone (SVZ) stem cell niche. White arrowheads in middle panel (Nes-ΔTK;PBS) indicate DCX-positive GFP-negative cells more distal in the RMS. White arrowheads in bottom panel (Nes-ΔTK;GCV) indicate GFP-positive/GFAP-positive but DCX-negative quiescent NSCs. d, Representative GFP immunostaining in sections from two untreated gliomas of Mut7;Nes-ΔTK mice. From tumor to tumor, varying numbers of GFP-positive cells were observed. e, Representative GFP and Ki67 co-immunostaining in two untreated gliomas of Mut7;Nes-ΔTK mice. White arrowheads highlight GFP-positive but Ki67-negative cells, demonstrating that many transgene GFP-positive cells are quiescent. f, Percentage of Ki67-positive cells in GFP-positive, GFP-negative, and overall tumor population in gliomas from untreated Mut7;Nes-ΔTK-GFP mice. g, Kaplan–Meier survival curve of untreated Mut7 and Mut7;Nes-ΔTK animals. No difference in percent survival was observed. h, Representative H&E staining of Mut7 and Mut7;Nes-ΔTK brains without treatment. Infiltrative malignant gliomas are present in the cortex (red arrowhead) of both genotypes. *, p<0.05; ***, p<0.001.