Abstract
Despite the evidence that there is a daily rhythm in smoking behavior and that the effects of drugs of abuse exhibit diurnal variations, very few studies have explored the extent to which sensitivity to the effects of nicotine vary over the course of the day. In the studies described in this report, the melatonin proficient mouse strain C3H/Ibg and the melatonin deficient mouse strains C57BL/6J and DBA/2J were assessed for diurnal variations in sensitivity to the effects of nicotine. Results indicated that there is significant variation in sensitivity to both activity and body temperature depressant effects of nicotine in the melatonin proficient C3H/Ibg strain with maximal sensitivity occurring during the latter third of the light period of the light cycle and minimal sensitivity taking place during the last third of the dark phase of the light cycle. The melatonin deficient strains did not exhibit diurnal differences in sensitivity to the effects of nicotine suggesting a potential role for melatonin in modulating the effects of nicotine. Experiments with knockout mice lacking both the Mtnr1a and Mtnr1b melatonin receptors confirmed that the reduced sensitivity observed during the dark phase is melatonin dependent. Diurnal variation in nicotinic receptor expression also was measured in cortex, hippocampus, hypothalamus and striatum using [125I] α-bungarotoxin and [125I]-epibatidine. [125I] α-bungarotoxin binding in hypothalamus of C3H mice exhibited a diurnal pattern with maximal binding observed in the latter third of the light portion of the light cycle. No other significant differences in binding were detected.
Keywords: nicotine, diurnal, melatonin, chronopharmacology, mice
1.1 Introduction
Cigarette smoking is the most common cause of preventable death in the United States. Approximately 440,000 deaths occur annually in the U.S. due to the development of tobacco-related illnesses, including cancer, stroke, and heart disease (Centers for Disease Control and Prevention (CDC) 2008). Despite the known health-risks associated with smoking, and the available treatments for smoking cessation, the vast majority of smokers are unable to quit (Centers for Disease Control and Prevention 2009). Clearly, a better understanding of the physiological effects of nicotine, the psychoactive agent in cigarette smoke, is needed.
Evidence from human studies suggests that nicotine administration follows a biological rhythm. Smokers show an increase in nicotine administration through cigarettes or gum during the morning hours, followed by a plateau in the afternoon, and a steep decline prior to sleep onset (Mooney et al. 2006). Not surprisingly, plasma levels of nicotine typically follow a similar pattern of fluctuation throughout the day, with levels rapidly increasing in the morning, stabilizing in the afternoon, and dropping at night (Benowitz et al. 1982). A recent study also has shown that smoking intensity varies over the course of the day with reduced smoking intensity observed during smoking episodes that occur during early morning hours (Grainge et al. 2009).
Several studies have demonstrated that sensitivity to the effects of drugs, including drugs of abuse, vary over the course of the day. For example, the psychomotor stimulant and reinforcing properties of cocaine are observed during the light but not dark phase of the light-dark cycle in most rodents (Akhisaroglu et al. 2004; Uz et al. 2002; Kurtuncu et al. 2004; Abarca et al. 2002). Therefore, the daily pattern of smoking in humans may be due to daily variations in sensitivity to the effects of nicotine. Some support for this possibility comes from a limited number of studies that have shown that rats display a greater sensitivity to the locomotor and hypothermic effects of nicotine during the light versus dark phase of a normal 12 hour light/12 hour dark cycle (Williams et al. 1993; Morley and Garner 1990). In addition, function and expression of α7 neuronal nicotinic receptors has been shown to vary in a time of day dependent manner. Markus et al. (2003) reported that α7 nicotinic acetylcholine receptor (nAChR) dependent nicotine-evoked [3H]glutamate release from rat cerebellar slices is greater during the dark phase of the light cycle. Seemingly contradictory to this finding, both Markus et al. (2003) and Morley and Garner (1990) reported that the expression of α7 nAChRs is reduced during nocturnal hours in several different brain regions, including the cerebellum.
Although the mechanism responsible for the observed diurnal variations in sensitivity to drugs of abuse is not entirely clear, melatonin appears to be critical for the reduced effects of cocaine during the dark phase of the light cycle. Manipulations that inhibit the dark phase surge of melatonin prevent conditioned place preference and locomotor sensitization to cocaine (Kurtuncu et al. 2004; Uz et al. 2003). Moreover, mouse strains such as C57BL/6, which possess naturally occurring mutations that significantly reduce their ability to synthesize melatonin, do not exhibit the diurnal pattern of cocaine sensitivity (Akhisaroglu et al. 2004; Uz et al. 2002). It has not been established whether melatonin is important for daily variations in sensitivity to nicotine. However, it has been demonstrated that the α7 nAChR dependent increase in nicotine-evoked [3H] glutamate release from rat cerebellar slices is abolished when pineal melatonin production is inhibited by either pharmacological disruption or by maintaining the animals in constant light (Markus et al., 2003). In addition, Lax (2008) demonstrated that physiological relevant levels of melatonin can inhibit excitatory post-synaptic potentials from non-α7 nAChRs in cultured cerebellar cells though a melatonin receptor-dependent mechanism. The importance of understanding the potential role of melatonin in modulating the effects of nicotine is highlighted by the finding that melatonin treatment reduces withdrawal-induced craving in abstinent smokers (Zhdanova and Piotrovskaya 2000).
Although there is some evidence that there is a time of day difference in nicotine sensitivity, the full extent of the time of day variability in nicotine sensitivity has not been determined. Moreover, the potential role of melatonin in modulating nicotine sensitivity has not been explored. The studies described in this report assessed sensitivity to the locomotor depressant and hypothermic effects of nicotine at 12 times points over the light/dark cycle to determine the extent to which sensitivity to an acute nicotine challenge varies over the course of the day. In addition, the regional expression of [125I] α-bungarotoxin and [125I]-epibatidine binding sites was measured at multiple time points. Finally, experiments were performed to establish whether melatonin signaling is essential for altered nicotine sensitivity during the dark phase of the light cycle.
1.2 Methods
1.2.1 Animals
Female and male C3H/Ibg and C3HMtnr1a/Mtnr1b double knockout mice and male C57BL/6J and DBA/2J mice were maintained on either a normal 12 h light/ 12 h dark cycle (lights on 0700 h to 1900 h) or a reverse 12 h dark/12 h light cycle (lights off 0700 h to 1900 h). C3HMtnr1a/Mtnr1b double knockout mice were generously provided by Dr. David Weaver (University of Massachusetts). Generation of these knockout mice was described elsewhere (Jin et al. 2003; Liu et al. 1997). Animals were maintained in the appropriate light cycle for at least 2 weeks prior to behavioral testing or sacrifice. Zeitgeber time (ZT) was defined relative to the light cycle (ZT hour 0: lights on, ZT hour 12: lights off). Animals had free access to food (Teklad 22/5 rodent diet, Harlan, Madison, WI) and water. All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Colorado's Institutional Animal Care and Use Committee. All efforts were made to minimize pain and suffering of animals and to minimize the use of animals.
1.2.2 Drugs
Nicotine free base was purchased from Sigma (St. Louis, MO, USA), prepared in sterile saline (0.9% NaCl) and administered by intraperitoneal injection (i.p.). Injection volume was 0.01ml/g body weight.
1.2.3 Y-maze and body temperature
Locomotor and rearing activity was determined in a symmetrical Y-maze. The maze consists of three arms which are 26 cm long, 6.1 cm wide, and 10.2 cm high and was constructed of red translucent acrylic plastic. Each arm is subdivided into two equal sections. Crosses from one section to another, as well as rears, were counted by photobeam detectors for 3 minutes. Body temperature was measured with a Bailey Instruments rectal probe. The probe was lubricated with peanut oil and was inserted 2.5 cm into the rectal cavity.
To investigate the main effect of nicotine on behavior, mice received an i.p. injection of physiological saline vehicle or nicotine dissolved in vehicle. Treatments were counterbalanced so that half the animals received nicotine during the initial test and the other half received saline during the initial test. Animals were tested under the opposite condition one week later. Each animal was tested for saline and nicotine effects at a single time point. Immediately following the injection, the animal was placed in a holding cage for 3 minutes, followed by 3 minutes of locomotor testing in the Y-maze. The mouse was then placed in a holding cage for another 7 minutes, followed by a body temperature measurement. These post-injection times have previously shown to correspond with a maximal response to nicotine (Marks et al. 1985). The testing of mice during the dark phase of the light cycle was done under dim red light (< 5 Lux). Initial experiments were conducted at zeitgeber times 8,9,10 and 11 (light period) and the 12 hr opposed times 20, 21,22, and 23. These initial times were chosen because they correspond to the hours that immediately precede sleep onset and awakening, respectively. Additional time points were added to provide a greater representation of the 24 hr daily period. The doses of nicotine tested for each mouse strain were based upon previously published data (Marks et al. 1989b). Results are reported as the nicotine response minus the saline response.
1.2.4 Tissue preparation for radioligand binding
Brains were removed from the skull following cervical dislocation, placed on an ice cold platform and the cortex, hippocampus, hypothalamus and striatum were dissected out. The brain regions were placed in ten volumes 0.1× Krebs Ringers Hepes (KRH) (11.8 mM NaCl; 0.48 mM KCl; 0.25 mM CaCl; 0.12 mM MgSO; and 2.0 mM HEPES pH 7.5) and homogenized using a teflon pestle. The homogenates were then incubated for 5 min at 37°C and subsequently centrifuged for 20 min at 18 000 × g. Following centrifugation, pellets were resuspended in ten volumes fresh 0.1× KRH and the above procedure repeated a total of four times. Samples were stored at −20°C as a pellet until use. On the day of receptor–ligand binding, pellets were resuspended in ten volumes of ice cold distilled water.
1.2.5 Ligand binding
The binding of epibatidine and α-bungarotoxin to brain membranes was measured essentially as described previously (Zambrano et al. 2009). Briefly, tissue homogenates were incubated with either 2nM [125I]-epibatidine or 2 nM [125I]-α-bungarotoxin (GE Healthcare, Arlington Heights, IL) in 30 μl of KRH (118 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 20 mM HEPES, pH = 7.4) at 24°C for epibatidine or 37°C for α-bungarotoxin for 4 h in 96-well microtiter plates. Non-specific binding was determined by the addition of 1 mM L-nicotine in the incubation. Samples were filtered through two filters, a Gelman type AE filter and a MFS GB100R, with a cell harvester (Inotech, Lansing MI). The filters were pre-soaked in 1× KRH and 0.5% polyethyleneimine prior to filtration. Individual filters were placed into 5-ml culture tubes and counted on a Packard Auto-Gamma 5000 gamma counter. Protein levels were measured by the method of Lowry et al. (Lowry et al. 1951).
1.2.6 Statistics
The effect of time and sex on baseline and nicotine-induced activity and body temperature in C3H mice were analyzed by multi-factoral regression analysis with time and sex as main factors. Nicotine sensitivity in C57BL/6J and DBA/2 mice and ligand binding across time were evaluated using One-way ANOVA. The effect of time on nicotine sensitivity in C3H Mtnr1a/Mtnr1b double knockout mice was done using Student's T-test.
1.3 Results
1.3.1 Time of day effects on baseline and nicotine-induced activity and body temperature in C3H mice
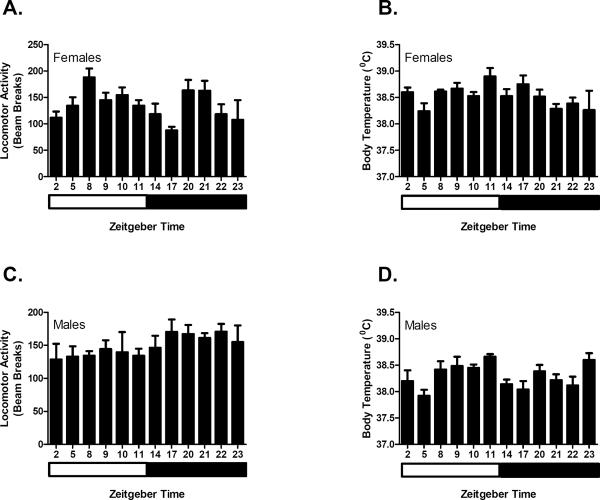
In order to investigate the time of day and sex effects on activity and body temperature under control conditions, Y-maze activity (combined locomotor and rearing activity) and body temperature were measured across 12 time points (ZT2, ZT5, ZT8, ZT9, Z10, ZT11, ZT14, ZT17, ZT20, ZT21, ZT22, ZT23) in both female and male C3H mice following a saline injection. C3H mice were chosen for these experiments because they are among the relatively few commonly used inbred mouse strains that exhibit diurnal variations in melatonin synthesis (Goto et al. 1989; Vivien-Roels et al. 1998). As shown in figure 1, there was a main effect of time (p < 0.005) but not sex on baseline Y-maze activity. There was a significant interaction between the two factors (p < 0.05). In contrast, there was a significant effect of time (p < 0.005) and sex (p < 0.005) on baseline body temperature but there was no significant interaction between these factors.
Figure 1.
Diurnal variation in activity and body temperature in C3H/Ibg mice following an i.p. saline injection. C3H/Ibg mice (n was between 4–9 animals per time point per sex) were injected with saline at 12 different time points across the light dark cycle and total activity (locomotor activity and rears) (A) and core body temperature (B) were determined. Results indicated that there was a time of day tested (p < 0.005) but not sex affect on total activity in a Y-maze while there was both a time of day tested (p < 0.005) and sex (p < 0.005) influence on core body temperature. Data are represented as mean ± SEM. White bar represents time frame of lights on and black bar represents time frame of lights off.
To more extensively characterize the effects of nicotine across the light-dark cycle, C3H mice were tested for the effects of a 1 mg/kg dose of nicotine on Y-maze activity and body temperature at each of the 12 time points throughout the light-dark cycle. The 1 mg/kg dose was chosen for initial experiments since it is near the ED50 value for the effects of nicotine on both locomotor activity and body temperature in C3H/Ibg mice (Marks et al. 1989b). Responses to nicotine were calculated as the difference in response between nicotine and saline injections (nicotine response – saline response) in order to control for non-nicotine dependent changes in activity and body temperature that occur over the course of the light-dark cycle. Results (figure 2) indicated a main effect of time for both nicotine-induced hypoactivity (p < 1 × 10−4) and hypothermia (p < 1.0 × 10−5). In general, nicotine sensitivity for both measures was minimal during the latter portion of the dark cycle (ZT20-23) and maximal during the latter period of the light cycle (ZT8-11). The time point where nicotine sensitivity differed from the greatest number of other time points was ZT10 (5 PM). Animals tested at this time point were more sensitive to the locomotor-depressant effects of nicotine than animals tested at all other time points except ZT5 and ZT8. Similarly, the ZT10 animals were more sensitive to the hypothermic effects of nicotine than animals tested at all other time points except ZT8, ZT9 and ZT11. Multi-factoral analysis indicated that there was neither a main effect of sex nor a significant interaction between time and sex for the time of day dependent effects of nicotine on activity and body temperature.
Figure 2.
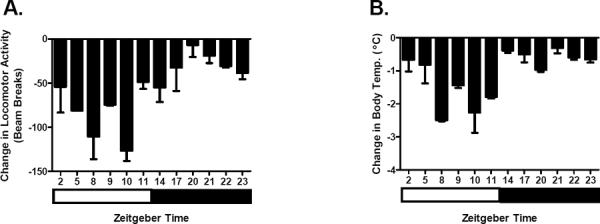
The depressant effect of nicotine on activity and body temperature is dependent upon the time of day. The effect of nicotine at each time point for activity (A) and body temperature (B) was determined by subtracting the nicotine response from the saline response (n was between 4–9 animals per time point per sex). A main effect of time of day on sensitivity to the depressant effects of nicotine on activity (p < 1 × 10−4) and body temperature (p < 1 × 10−5) was observed. In general, maximal sensitivity to nicotine for both measures occurred during the light phase and minimal sensitivity occurred during the dark phase of the light cycle. Data are represented as mean ± SEM. White bar represents time frame of lights on and black bar represents time frame of lights off.
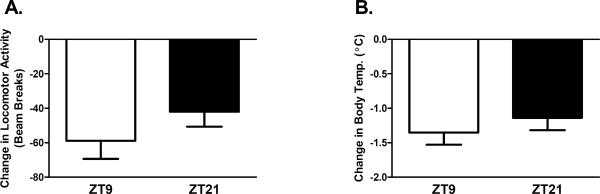
To explore dose-dependent effects on daily variations in nicotine sensitivity, additional male C3H mice were tested at 0.5 mg/kg and 1.5 mg/kg nicotine on Y-maze activity and body temperature at ZT9 and ZT21. Results (Figure 3) indicated that the effect of time of day on nicotine-induced hypolocomotion exhibits a small dose range where significant time of day differences are only seen at the original 1 mg/kg dose (p < 0.05). In contrast, time of day effects on nicotine-induced hypothermia is seen across multiple doses of nicotine (p < 0.05 at 0.5 mg/kg, and p < 0.005 at 1 mg/kg).
Figure 3.
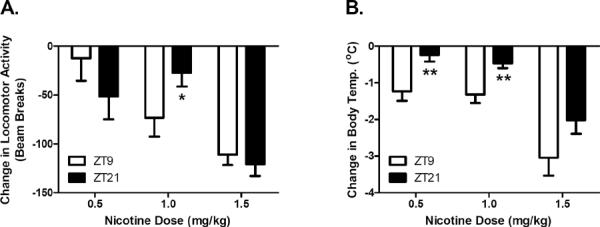
Time of day effects of nicotine are dose dependent. Male C3H/Ibg were tested for the effect of 3 different doses of nicotine on activity and body temperature at ZT9 and ZT21 (n was between 5–13 animals per dose per time point). Time of day dependent effects of nicotine on activity were only seen at the 1.0 mg/kg dose (p < 0.05) while significant time of day effects of nicotine on body temperature were observed at the 0.5 (p < 0.01) and 1.0 mg/kg nicotine doses (p < 0.01). The time of day-dependent effect of a 1.5 mg/kg dose of nicotine on body temperature approached significance (p = 0.054). Data are represented as mean ± SEM. * = p < 0.05; ** = p < 0.01.
1.3.2 Potential role of melatonin in modulating light-dark differences in nicotine sensitivity
Several studies in rodents have suggested that light cycle related differences in sensitivity to drugs of abuse are dependent, in part, on the synthesis of melatonin during the dark phase of the light-dark cycle (Kurtuncu et al. 2004; Uz et al. 2003). In C3H mice, melatonin levels are elevated during the latter part of the dark cycle (Goto et al. 1989; Vivien-Roels et al. 1998; Welp et al. 2010), the period of the day where sensitivity to the effects of nicotine was at a minimum. To explore the potential role of melatonin in modulating time of day sensitivity to nicotine, two additional mouse strains, C57BL/6J and DBA/2Ibg, were tested for the time of day dependent effects of nicotine on activity and body temperature. Due to genetic defects in enzymes required for melatonin synthesis, a nocturnal surge in melatonin synthesis is significantly blunted in these two mouse strains (Kasahara et al. 2010; Goto et al. 1989; Roseboom et al. 1998; Welp et al. 2010). C57BL/6J mice were tested at three doses of nicotine (0.25 mg/kg, 0.5 mg/kg, and 1.0 mg/kg), at ZT21, a time when C3H mice have elevated levels of melatonin and show reduced sensitivity to nicotine, and ZT9, the time point 12 hr opposite ZT21. Lower doses of nicotine were used in C57BL/6J mice since they are more sensitive to the hypothermic and locomotor depressant effects of nicotine (Marks et al. 1989b). No significant time of day differences in nicotine sensitivity were observed in C57BL/6J mice at any of the tested doses (figure 4 A,B). Likewise, DBA/2Ibg mice did not differ in nicotine sensitivity when tested at ZT9 and ZT21 at three different doses of nicotine (0.5 mg/kg, 1.0 mg/kg, and 1.5 mg/kg) (figure 4 C, D). The finding that these melatonin deficient mouse strains do not exhibit time of day differences in nicotine sensitivity supports a possible role of melatonin in modulating time of day-dependent effects of nicotine.
Figure 4.
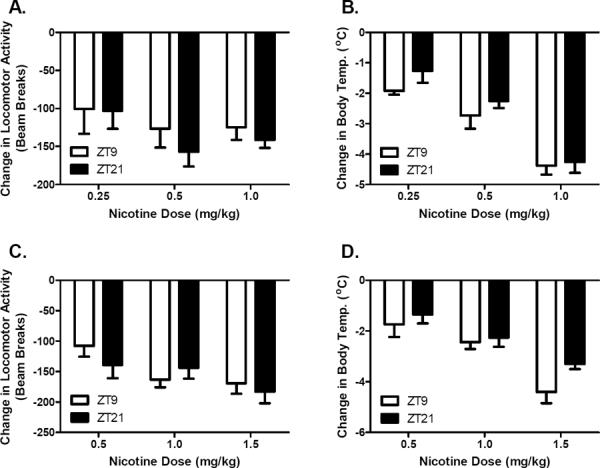
Melatonin deficient mouse strains do not differ in sensitivity to the effects of nicotine between ZT9 and ZT21. C57BL/6J male mice exhibited similar sensitivity to the effects of nicotine on activity (A) and body temperature (B) at ZT9 and ZT21 when tested at either 0.25 (n = 5 per time point) or 0.5 mg/kg (n = 12–13 per time point) nicotine. Similarly, 0.5 mg/kg (n = 5–8 per time point) and 1.0 mg/kg (n = 12–14 per time point) doses of nicotine did not have zeitgeber time-dependent effects on activity (C) or body temperature (D) in DBA/2J male mice. Data are represented as mean ± SEM.
To further assess the potential relationship between melatonin and sensitivity to nicotine, mice proficient at melatonin synthesis (C3H genetic background) but lacking the melatonin receptors Mtnr1a and Mtnr1b were tested for the effects of a 1 mg/kg dose of nicotine on activity and body temperature at a time point in the dark where melatonin levels are high and sensitivity to nicotine is minimal (ZT21) and a time point during the light phase of the light cycle that is 12 hr opposite (ZT9). Results (figure 5) indicated that the time-dependent difference in sensitivity to either nicotine-induced depression of activity or body temperature is abolished in the absence of Mtnr1a and Mtnr1b-dependent melatonin signaling. This observation supports a role for melatonin in regulating sensitivity to nicotine.
Figure 5.
Melatonin receptors are essential for reduced sensitivity to nicotine at ZT21. C3H genetic background mice that possess homozygous null mutations for the melatonin receptors Mtnr1a and Mtnr1b are not differentially sensitive to the effects of 1 mg/kg nicotine on activity (A) or body temperature (B) at ZT9 and ZT21 (n = 24 per time point).
1.3.3 Time of day effects on nicotinic receptor levels
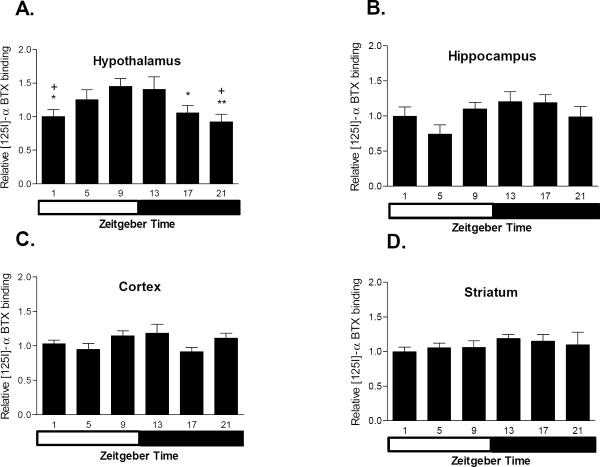
Previous data have indicated that there is a time of day difference in levels of α7 nAChR expression, as measured by [125I]-α-bungarotoxin (αBTX), in the cerebellum (Markus et al. 2003) and hypothalamus (Morley and Garner 1990) of rat brain. Therefore, αBTX levels were measured in four brain regions from C3H mice across 6 time points of the light-dark cycle (figure 6). The four brain regions examined were cortex, hypothalamus, hippocampus and striatum. Cortex and hypothalamus were chosen to correspond to the regions previously assessed by Morley and Garner (1990). The hippocampus and striatum also were assessed because these two brain regions are known to express melatonin receptors (Zisapel et al. 1988; Musshoff et al. 2002; Uz et al. 2005). Similar to previous findings, there were significant differences in αBTX levels in the hypothalamus over the light-dark cycle. The nadir of αBTX binding occurred during the late period of the dark cycle and the peak of αBTX binding occurred during the late light/early dark period of the light-dark cycle. There were no light cycle dependent differences in αBTX binding in cortex, hippocampus or striatum. In addition, levels of high affinity nAChRs, as measured by [125I] epibatidine binding, did not vary over the light-dark cycle in any of the four regions examined (Table 1). Levels of αBTX and [125I]epibatidine binding also were measured in the same brain regions in the melatonin deficient mouse strain DBA/2Ibg. No time of day variation in the levels of either αBTX or [125I]epibatidine binding were observed in any of the brain regions from DBA/2Ibg mice (Table 1).
Figure 6.
The effect of time of day on levels of [125I]-α-bungarotoxin binding is brain region specific. [125I]-α-bungarotoxin binding was measured at 6 time points and 4 brain regions (n = 6–7 per brain region per time point). Results indicated that there is a daily variation in [125I]-α-bungarotoxin binding in the hypothalamus (p < 0.05) with maximal levels occurring between 2 hr before lights out and 2 hr after lights out. There was no effect of time of day in the remaining 3 brain regions. Data are represented as mean ± SEM. White bar represents time frame of lights on and black bar represents time frame of lights off. * = p < 0.05 relative to ZT 9; ** = p < 0.01 relative to ZT9; + = p < 0.05 relative to ZT 13.
TABLE 1.
Binding data for [125I]-α-bungarotoxin (BTX) and [125I] epibatidine (Epi) across 6 times points and 4 brain regions in C3H/Ibg and DBA/2 mice. Data were normalized to ZT1 (7 AM) and are represented as mean ± SEM. Significant effects of time of day were only observed for BTX binding in the hypothalamus of C3H mice (see figure 6 for details).
| Brain Region | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortex | Hippocampus | Hypothalamus | Striatum | ||||||||||||||||||||||
| Time Point (ZT) | Time Point (ZT) | Time Point (ZT) | Time Point (ZT) | ||||||||||||||||||||||
| Strain | Ligancl | 1 | 5 | 9 | 13 | 17 | 21 | 1 | 5 | 9 | 13 | 17 | 21 | 1 | 5 | 9 | 13 | 17 | 21 | 1 | 5 | 9 | 13 | 17 | 21 |
| C3H | BTX | 1.00 (0.06) | 0.95 (0.09) | 1.14 (0.07) | 1.18 (0.13) | 0.91 (0.06) | 1.11 (0.08) | 1.00 (0.13) | 0.74 (0.13) | 1.10 (0.09) | 1.20 (0.14) | 1.19 (0.11) | 0.98 (0.15) | 1.00 (0.10) | 1.25 (0.15) | 1.45 (0.12) | 1.41 (0.19) | 1.06 (0.11) | 0.92 (0.11) | 1.00 (0.07) | 1.05 (0.07) | 1.06 (0.10) | 1.19 (0.06) | 1.15 (0.09) | 1.10 (0.18) |
| Epi | 1.00 (0.12) | 1.08 (0.07) | 0.77 (0.15) | 1.02 (0.04) | 1.13 (0.07) | 0.84 (0.09) | 1.00 (0.09) | 0.85 (0.08) | 0.83 (0.09) | 0.91 (0.11) | 0.91 (0.10) | 0.78 (0.11) | 1.00 (0.09) | 0.97 (0.09) | 0.94 (0.09) | 0.93 (0.10) | 0.97 (0.10) | 0.66 (0.08) | 1.00 (0.05) | 0.99 (0.06) | 0.80 (0.16) | 0.92 (0.16) | 1.06 (0.07) | 1.16 (0.16) | |
| DBA | BTX | 1.00 (0.05) | 0.99 (0.09) | 1.02 (0.05) | 0.93 (0.11) | 1.19 (0.12) | 0.99 (0.08) | 1.00 (0.16) | 0.91 (0.08) | 0.93 (0.10) | 1.02 (0.15) | 0.86 (0.11) | 0.95 (0.14) | 1.00 (0.08) | 0.91 (0.10) | 0.96 (0.06) | 0.92 (0.16) | 0.89 (0.07) | 0.82 (0.07) | 1.00 (0.09) | 1.08 (0.09) | 0.77 (0.04) | 1.14 (0.11) | 1.04 (0.09) | 1.05 (0.06) |
| Epi | 1.00 (0.05) | 1.25 (0.09) | 1.18 (0.08) | 1.14 (0.07) | 1.02 (0.04) | 1.05 (0.05) | 1.00 (0.06) | 1.23 (0.05) | 1.01 (0.08) | 1.22 (0.30) | 1.33 (0.16) | 0.92 (0.13) | 1.00 (0.13) | 0.86 (0.14) | 0.80 (0.06) | 0.89 (0.13) | 0.80 (0.05) | 0.93 (0.09) | 1.00 (0.06) | 1.05 (0.07) | 0.91 (0.10) | 1.01 (0.08) | 1.00 (0.09) | 0.94 (0.05) | |
1.4 Discussion
The effects of many drugs of abuse are known to vary over the course of the day, yet very little work has been done to examine the daily variations in sensitivity to the effects of nicotine, one of the most commonly consumed psychotropic drugs. The data reported here demonstrate that there is significant diurnal variation in sensitivity to the hypothermic and activity depressing effects of nicotine. Relative to the light/dark cycle, maximal sensitivity occurred during the latter half of the light phase and minimal sensitivity occurred during the latter half of the dark cycle. The heightened sensitivity to nicotine during the light phase of the light/dark cycle is consistent with the findings of Williams et al. (Williams et al. 1993) who found nicotine-induced hypothermia in rats to be greater during the light phase of the light/dark cycle. Of particular relevance is the finding that even during the period of the day where most experimental procedures are conducted (8AM to 6 PM), there is significant variability in sensitivity to nicotine. This time of day dependent effect of nicotine likely contributes to inter-and intra-laboratory variability and suggests that time of day should be considered carefully when assessing the effects of nicotine, particularly when using melatonin-competent mouse strains such as C3H or CBA.
The reduced sensitivity to nicotine observed during the dark phase of the light cycle occurred during the peak of melatonin synthesis and was found to be dependent upon the presence of the melatonin receptors Mtnr1a and Mtnr1b. In the absence of these two major melatonin receptors, nicotine sensitivity at ZT21 (4 AM relative time) did not differ from nicotine sensitivity at ZT9 (4 PM). This finding demonstrates that melatonin signaling is necessary for the reduced sensitivity to nicotine that is seen during the latter stages of the dark phase of the light cycle. However, it remains to be determined whether one or both melatonin receptors are necessary for the diurnal variation in nicotine sensitivity. Nonetheless, these data are consistent with previous studies that have shown that diurnal variation in sensitivity to other drugs of abuse may be melatonin dependent. For example, C3H mice develop conditioned place preference (Kurtuncu et al. 2004) and locomotor sensitization (Uz et al. 2003) to cocaine during the light but not dark phase of the light-dark cycle. However, following pinealectomy (i.e. no melatonin synthesis), C3H mice develop conditioned place preference and locomotor sensitization to cocaine during both phases of the light-dark cycle. This diurnal difference in sensitivity to cocaine appears to be restricted to rodents with normal rhythms of melatonin synthesis (Akhisaroglu et al. 2004) further supporting a role of melatonin in regulating the daily variations in sensitivity to drugs of abuse. Like cocaine, the effects of nicotine appear to be diminished during the dark phase of the light-dark cycle in a melatonin-dependent manner. This observation is interesting in light of the observation that daily smoking begins to decline in humans around the time that melatonin levels begin to rise (Mooney et al. 2006).
The mechanism through which melatonin modulates nicotine sensitivity is not known. However, a limited number of studies have shown that the function and/or expression of nicotinic receptors in various tissues and cell types is altered by melatonin. For example, Schiller et al. (2003) found that melatonin inhibits nicotinic receptor (nAChR) function in the rat pheochromocytoma cell line, PC12 with an IC50 of 8.6 μM. In addition, Markus et al. (2003) reported that α7 nicotinic receptor-dependent glutamate release in the rat cerebellum is greater during the dark phase of the light cycle in a melatonin dependent manner. This group also found that α7-containing nAChRs show a circadian pattern of expression in which there are reduced receptor levels in the cerebellum during the dark period. Although it is not entirely clear how reduced numbers of α7 nAChRs leads to increased α7 nAChR-dependent glutamate release, the observation that α7 nAChRs are reduced during the dark phase is consistent with the data reported in this manuscript as well as by data described in a study by Morley and Garner (1990). Both the current study and the study by Morley and Garner demonstrated that α7 nAChRs are reduced in the hypothalamus during the dark phase. The diurnal variation in expression of the α7 nAChR detected in the current study was not observed in three other brain areas examined. In addition, there was no detectable variation in the expression of high affinity nAChRs across all four brain regions examined. Thus, diurnal variation in nAChR expression appears to be subtype and brain region specific. Other studies have suggested that α7 nAChRs are not involved in regulating nicotine's effects on activity and body temperature (Marks et al. 1989a; Marks et al. 1989b; Tritto et al. 2004). However, these studies were mainly performed with mouse strains that do not exhibit diurnal variations in melatonin synthesis and were conducted only during the light portion of the light cycle. Thus, it would be worthwhile to assess the role of α7 nAChRs in modulating the locomotor and body temperature depressant effects of nicotine during different times of the day in inbred mouse strains and α7 nAChR knockout mice that exhibit normal diurnal melatonin production.
Another mechanism though which melatonin might modulate the effects of nicotine is by inhibiting protein kinase A signaling pathways including CREB activation. Nicotine has been shown to increase CREB activity in vitro and in vivo via several nAChR subtypes (Lenz et al. 2010; Pluzarev and Pandey 2004; Hou et al. 2004; Brunzell et al. 2003; Hu et al. 2002; Nakayama et al. 2001) and activation of CREB seems to be essential for some behavioral responses to nicotine including conditioned place preference (Pascual et al. 2009; Brunzell et al. 2009). In contrast, melatonin signaling through Mtnr1a and/or Mtnr1b typically inhibits the protein kinase A signaling pathway, including reducing CREB activity via pertussis toxin sensitive Gi proteins (Hardeland 2009). Thus, elevated levels of melatonin may suppress the ability of nicotine to activate CREB or other PKA dependent cellular components and consequently diminish the effects of nicotine. Cocaine reinforcement also is dependent upon CREB (Choi et al. 2006) and is attenuated by melatonin (Akhisaroglu et al. 2004; Kurtuncu et al. 2004; Uz et al. 2003; Uz et al. 2002) suggesting that the inhibitory effect of melatonin on the effects of these drugs of abuse may be through a common mechanism.
The effect of melatonin signaling on nicotine sensitivity also could be indirect. For example, it has been shown that most components of the dopaminergic system possess a diurnal rhythm with dopaminergic activity at a nadir during peak melatonin synthesis (Khaldy et al. 2002). Other neurotransmitter systems including acetylcholine, GABA, glutamate and serotonin also exhibit circadian variations in activity (Day et al. 1991; Rueter and Jacobs 1996; Marquez de et al. 2000; Castaneda et al. 2004). Therefore, it is plausible that the time of day dependent effects of nicotine are due, in part, to daily variations in the activity of one or more neurotransmitter systems.
In summary, the data described in this manuscript confirm and extend the observation that the acute effects of nicotine on locomotion and body temperature are dependent upon the time of day. In general, the nadir of sensitivity to nicotine occurs during the latter portion of the dark phase of the light cycle just prior to sleep onset at a time when melatonin levels are elevated. In contrast, the peak of nicotine sensitivity occurs during the latter stages of the light phase of the light cycle in the period preceding awakening. The reduced sensitivity to nicotine seen during the dark phase appears to be dependent upon melatonin as deletion of the melatonin receptors Mtnr1a and Mtnr1b eliminates the diurnal variation in nicotine sensitivity. Interestingly, the nadir and peak of nicotine sensitivity in mice with diurnal variations in melatonin synthesis correspond to the times of day in humans when smoking begins to decline or when there is heightened sensitivity to the subjective effects of nicotine, respectively. Thus, daily variations in sensitivity to the effects of nicotine may contribute to daily smoking patterns in humans and reduced smoking in the evening may be influenced by increased melatonin levels. Understanding these time of day differences in nicotine sensitivity and how they might contribute to smoking behavior may provide novel insight into the underlying neurobiology of nicotine addiction.
Acknowledgements
The authors would like to thank Ms. Hannah Gissel and Ms. Lori Fraser for their technical assistance. Support for this project was provided by DA022462, DA017637 and DA015663.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhisaroglu M, Ahmed R, Kurtuncu M, Manev H, Uz T. Diurnal rhythms in cocaine sensitization and in Period1 levels are common across rodent species. Pharmacol Biochem Behav. 2004;79:37–42. doi: 10.1016/j.pbb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Benowitz NL, Kuyt F, Jacob P., III Circadian blood nicotine concentrations during cigarette smoking. Clin Pharmacol Ther. 1982;32:758–764. doi: 10.1038/clpt.1982.233. [DOI] [PubMed] [Google Scholar]
- 4.Brunzell DH, Mineur YS, Neve RL, Picciotto MR. Nucleus accumbens CREB activity is necessary for nicotine conditioned place preference. Neuropsychopharmacology. 2009;34:1993–2001. doi: 10.1038/npp.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunzell DH, Russell DS, Picciotto MR. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem. 2003;84:1431–1441. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 6.Castaneda TR, de Prado BM, Prieto D, Mora F. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res. 2004;36:177–185. doi: 10.1046/j.1600-079x.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Cigarette Smoking Among Adults and Trends in Smoking Cessation --- United States, 2008. Morbidity and Mortality Weekly Report. 2009;58:1227–1232. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Smoking-attributable mortality, years of potential life lost, and productivity losses, United States, 2000--2004. Morbidity and Mortality Weekly Report. 2008;57:1226–1228. [PubMed] [Google Scholar]
- 9.Choi KH, Whisler K, Graham DL, Self DW. Antisense-induced reduction in nucleus accumbens cyclic AMP response element binding protein attenuates cocaine reinforcement. Neuroscience. 2006;137:373–383. doi: 10.1016/j.neuroscience.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 10.Day J, Damsma G, Fibiger HC. Cholinergic activity in the rat hippocampus, cortex and striatum correlates with locomotor activity: an in vivo microdialysis study. Pharmacol Biochem Behav. 1991;38:723–729. doi: 10.1016/0091-3057(91)90233-r. [DOI] [PubMed] [Google Scholar]
- 11.Goto M, Oshima I, Tomita T, Ebihara S. Melatonin content of the pineal gland in different mouse strains. J Pineal Res. 1989;7:195–204. doi: 10.1111/j.1600-079x.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 12.Grainge MJ, Shahab L, Hammond D, O'Connor RJ, McNeill A. First cigarette on waking and time of day as predictors of puffing behaviour in UK adult smokers. Drug Alcohol Depend. 2009 doi: 10.1016/j.drugalcdep.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Hardeland R. Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors. 2009;35:183–192. doi: 10.1002/biof.23. [DOI] [PubMed] [Google Scholar]
- 14.Hou J, Kuromi H, Fukasawa Y, Ueno K, Sakai T, Kidokoro Y. Repetitive exposures to nicotine induce a hyper-responsiveness via the cAMP/PKA/CREB signal pathway in Drosophila. J Neurobiol. 2004;60:249–261. doi: 10.1002/neu.20021. [DOI] [PubMed] [Google Scholar]
- 15.Hu M, Liu QS, Chang KT, Berg DK. Nicotinic regulation of CREB activation in hippocampal neurons by glutamatergic and nonglutamatergic pathways. Mol Cell Neurosci. 2002;21:616–625. doi: 10.1006/mcne.2002.1202. [DOI] [PubMed] [Google Scholar]
- 16.Jin X, von GC, Pieschl RL, Gribkoff VK, Stehle JH, Reppert SM, et al. Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol. 2003;23:1054–1060. doi: 10.1128/MCB.23.3.1054-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasahara T, Abe K, Mekada K, Yoshiki A, Kato T. Genetic variation of melatonin productivity in laboratory mice under domestication. Proc Natl Acad Sci U S A. 2010;107:6412–6417. doi: 10.1073/pnas.0914399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khaldy H, Leon J, Escames G, Bikjdaouene L, Garcia JJ, Acuna-Castroviejo D. Circadian rhythms of dopamine and dihydroxyphenyl acetic acid in the mouse striatum: effects of pinealectomy and of melatonin treatment. Neuroendocrinology. 2002;75:201–208. doi: 10.1159/000048238. [DOI] [PubMed] [Google Scholar]
- 19.Kurtuncu M, Arslan AD, Akhisaroglu M, Manev H, Uz T. Involvement of the pineal gland in diurnal cocaine reward in mice. Eur J Pharmacol. 2004;489:203–205. doi: 10.1016/j.ejphar.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Lax P. Melatonin inhibits nicotinic currents in cultured rat cerebellar granule neurons. J Pineal Res. 2008;44:70–77. doi: 10.1111/j.1600-079X.2007.00481.x. [DOI] [PubMed] [Google Scholar]
- 21.Lenz B, Klafki HW, Hillemacher T, Killisch N, Schaller G, Frieling H, et al. Smoking behaviour is associated with expression and phosphorylation of CREB in human buffy coat. Int J Neuropsychopharmacol. 2010;13:207–215. doi: 10.1017/S1461145709991052. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Marks MJ, Romm E, Bealer SM, Collins AC. A test battery for measuring nicotine effects in mice. Pharmacol Biochem Behav. 1985;23:325–330. doi: 10.1016/0091-3057(85)90577-5. [DOI] [PubMed] [Google Scholar]
- 25.Marks MJ, Romm E, Campbell SM, Collins AC. Variation of nicotinic binding sites among inbred strains. Pharmacol Biochem Behav. 1989a;33:679–689. doi: 10.1016/0091-3057(89)90407-3. [DOI] [PubMed] [Google Scholar]
- 26.Marks MJ, Stitzel JA, Collins AC. Genetic influences on nicotine responses. Pharmacol Biochem Behav. 1989b;33:667–678. doi: 10.1016/0091-3057(89)90406-1. [DOI] [PubMed] [Google Scholar]
- 27.Markus RP, Santos JM, Zago W, Reno LA. Melatonin nocturnal surge modulates nicotinic receptors and nicotine-induced [3H]glutamate release in rat cerebellum slices. J Pharmacol Exp Ther. 2003;305:525–530. doi: 10.1124/jpet.102.045625. [DOI] [PubMed] [Google Scholar]
- 28.Marquez de PB, Castaneda TR, Galindo A, del AA, Segovia G, Reiter RJ, et al. Melatonin disrupts circadian rhythms of glutamate and GABA in the neostriatum of the aware rat: a microdialysis study. J Pineal Res. 2000;29:209–216. doi: 10.1034/j.1600-0633.2002.290403.x. [DOI] [PubMed] [Google Scholar]
- 29.Mooney M, Green C, Hatsukami D. Nicotine self-administration: cigarette versus nicotine gum diurnal topography. Hum Psychopharmacol. 2006;21:539–548. doi: 10.1002/hup.808. [DOI] [PubMed] [Google Scholar]
- 30.Morley BJ, Garner LL. Light-dark variation in response to chronic nicotine treatment and the density of hypothalamic alpha-bungarotoxin receptors. Pharmacol Biochem Behav. 1990;37:239–245. doi: 10.1016/0091-3057(90)90328-f. [DOI] [PubMed] [Google Scholar]
- 31.Musshoff U, Riewenherm D, Berger E, Fauteck JD, Speckmann EJ. Melatonin receptors in rat hippocampus: molecular and functional investigations. Hippocampus. 2002;12:165–173. doi: 10.1002/hipo.1105. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama H, Numakawa T, Ikeuchi T, Hatanaka H. Nicotine-induced phosphorylation of extracellular signal-regulated protein kinase and CREB in PC12h cells. J Neurochem. 2001;79:489–498. doi: 10.1046/j.1471-4159.2001.00602.x. [DOI] [PubMed] [Google Scholar]
- 33.Pascual MM, Pastor V, Bernabeu RO. Nicotine-conditioned place preference induced CREB phosphorylation and Fos expression in the adult rat brain. Psychopharmacology (Berl) 2009;207:57–71. doi: 10.1007/s00213-009-1630-4. [DOI] [PubMed] [Google Scholar]
- 34.Pluzarev O, Pandey SC. Modulation of CREB expression and phosphorylation in the rat nucleus accumbens during nicotine exposure and withdrawal. J Neurosci Res. 2004;77:884–891. doi: 10.1002/jnr.20216. [DOI] [PubMed] [Google Scholar]
- 35.Roseboom PH, Namboodiri MA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, et al. Natural melatonin 'knockdown' in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res. 1998;63:189–197. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- 36.Rueter LE, Jacobs BL. Changes in forebrain serotonin at the light-dark transition: correlation with behaviour. Neuroreport. 1996;7:1107–1111. doi: 10.1097/00001756-199604100-00031. [DOI] [PubMed] [Google Scholar]
- 37.Schiller ED, Champney TH, Reiter CK, Dohrman DP. Melatonin inhibition of nicotine-stimulated dopamine release in PC12 cells. Brain Res. 2003;966:95–102. doi: 10.1016/s0006-8993(02)04200-2. [DOI] [PubMed] [Google Scholar]
- 38.Tritto T, McCallum SE, Waddle SA, Hutton SR, Paylor R, Collins AC, et al. Null mutant analysis of responses to nicotine: deletion of beta2 nicotinic acetylcholine receptor subunit but not alpha7 subunit reduces sensitivity to nicotine-induced locomotor depression and hypothermia. Nicotine Tob Res. 2004;6:145–158. doi: 10.1080/14622200310001656966. [DOI] [PubMed] [Google Scholar]
- 39.Uz T, Akhisaroglu M, Ahmed R, Manev H. The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology. 2003;28:2117–2123. doi: 10.1038/sj.npp.1300254. [DOI] [PubMed] [Google Scholar]
- 40.Uz T, Arslan AD, Kurtuncu M, Imbesi M, Akhisaroglu M, Dwivedi Y, et al. The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Brain Res Mol Brain Res. 2005;136:45–53. doi: 10.1016/j.molbrainres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Uz T, Javaid JI, Manev H. Circadian differences in behavioral sensitization to cocaine: putative role of arylalkylamine N-acetyltransferase. Life Sci. 2002;70:3069–3075. doi: 10.1016/s0024-3205(02)01559-x. [DOI] [PubMed] [Google Scholar]
- 42.Vivien-Roels B, Malan A, Rettori MC, Delagrange P, Jeanniot JP, Pevet P. Daily variations in pineal melatonin concentrations in inbred and outbred mice. J Biol Rhythms. 1998;13:403–409. doi: 10.1177/074873098129000228. [DOI] [PubMed] [Google Scholar]
- 43.Welp A, Manz B, Peschke E. Development and validation of a high throughput direct radioimmunoassay for the quantitative determination of serum and plasma melatonin (N-acetyl-5-methoxytryptamine) in mice. J Immunol Methods. 2010;358:1–8. doi: 10.1016/j.jim.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 44.Williams RL, Soliman KF, Mizinga KM. Circadian variation in tolerance to the hypothermic action of CNS drugs. Pharmacol Biochem Behav. 1993;46:283–288. doi: 10.1016/0091-3057(93)90354-v. [DOI] [PubMed] [Google Scholar]
- 45.Zambrano CA, Marks MJ, Cassels BK, Maccioni RB. In vivo effects of 3-iodocytisine: pharmacological and genetic analysis of hypothermia and evaluation of chronic treatment on nicotinic binding sites. Neuropharmacology. 2009;57:332–342. doi: 10.1016/j.neuropharm.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhdanova IV, Piotrovskaya VR. Melatonin treatment attenuates symptoms of acute nicotine withdrawal in humans. Pharmacol Biochem Behav. 2000;67:131–135. doi: 10.1016/s0091-3057(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 47.Zisapel N, Nir I, Laudon M. Circadian variations in melatonin-binding sites in discrete areas of the male rat brain. FEBS Lett. 1988;232:172–176. doi: 10.1016/0014-5793(88)80411-3. [DOI] [PubMed] [Google Scholar]