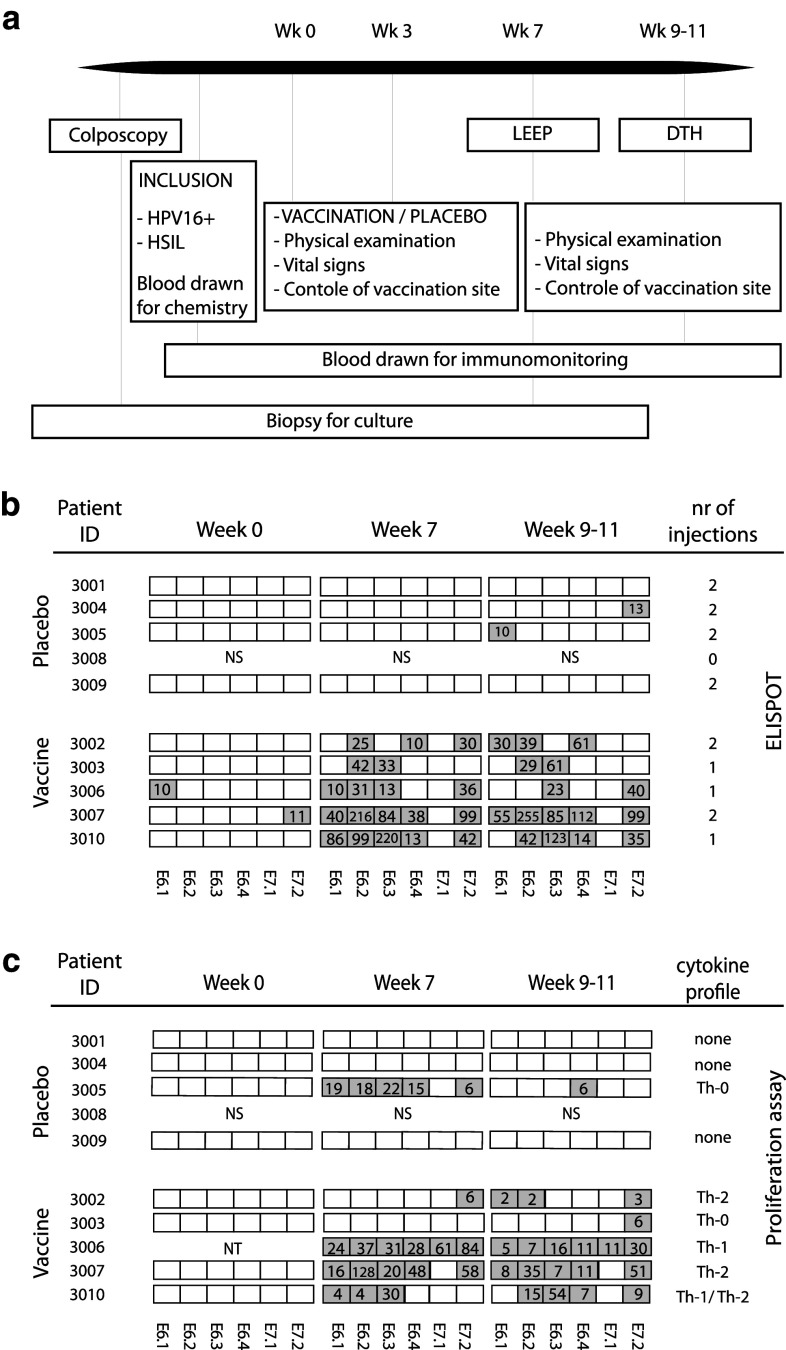
Fig. 1.
Schematic representation of the placebo-controlled randomized trial and summary of immunological results. a Patients were recruited at colposcopy visit. After informed consent, HPV testing was performed by PCR and an extra biopsy was taken for the culture of HSIL-infiltrating lymphocytes. Patients with histological proven HPV16+ HSIL then consented to the vaccination study at which time blood was drawn for chemistry and base-line immunomonitoring. Patients in arm 1 received the vaccine at a dose of 300 μg per peptide twice with a 3-week interval; patients in arm 2 received a placebo (PBS). Seven weeks after the first vaccination, a LEEP excision was performed at which time an extra biopsy of the HSIL was taken and blood drawn for immunomonitoring. DTH skin test was performed 2–4 weeks after surgery at which time blood was drawn to measure the effect of the LEEP excision on the systemic immune response. b Immunological results of all the patients using PBMC from three different time points. Week 0 (prevaccination), week 7 (post-vaccination) and week 9–11 (after LEEP excision). Systemic HPV16-specific T-cell reactivity against six peptide pools (4 E6 and 2 E7 peptide pools) was determined by IFNγ-ELISPOT. The boxes in gray show the number of HPV-specific IFNγ-producing T cells per 100,000 cells. c HPV specificity determined by the proliferation assay (LST). The gray boxes indicate the (stimulation index) SI of the HPV-specific proliferative responses. The culture at week 0 of patient 3006 was not tested due to technical problems. To the right, the overall cytokine profile based on the outcome of tested supernatants of the LST by cytokine bead array (CBA) is indicated. A Th-0 response indicates weak cytokine production inconclusive for a Th-1 or Th-2 response. Patient 3008 was randomized, but never showed up for vaccination; NS, not started