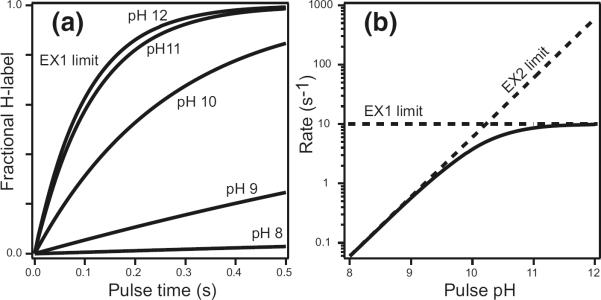
Figure 2.
HX of protected amides. The additional protection afforded by H-bonded structure depends on the thermodynamic and kinetic parameters of transient opening reactions that separate the H-bond and expose the hydrogen to solvent. Exchange rate increases linearly with solvent catalyst concentration in the EX2 range but will ultimately limit at the structural opening rate (EX1 limit). In the EX2 region, the reference chemical rate (eq 1, Figure 1) is decreased by the fraction of time that the hydrogen is exposed, given by kop/(kop + kcl) ≈ Kop (from eq 2), therefore can indicate the free-energy of the responsible structural opening reaction (eq 3). In the EX1 region, the HX rate becomes equal to, and therefore provides a way to measure, the rate for the responsible structural opening (eq 4). EX1 behavior can be documented by its insensitivity to pH as shown here or by correlated MS behavior when multiple amides experience the same EX1 exchange rate so that MS results show a molecular species moving from the unlabeled mass to the fully labeled mass in one concerted step.