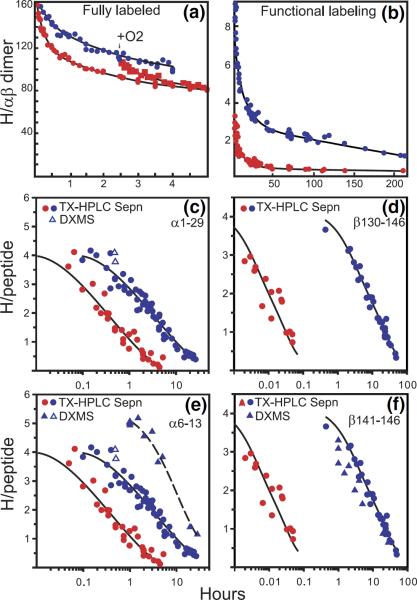
Figure 3.
HX results for hemoglobin (Hb) illustrating the progression of HX technology. (a) Whole Hb initially labeled to exchange equilibrium in tritiated water, then exchanged-out in oxy (red) and deoxy (blue) forms, measured by the early tritiumgel-filtration method [36]. Some allosterically sensitive sites are faster in oxyHb. When O2 is added to exchanging deoxy Hb, the sensitive sites are made faster and the separate curves merge. (b) Functional labeling results for whole Hb with exchange-in for only 1 min (pH 7.4, 0 °C [37]). The functional labeling method selectively labels some allosterically sensitive sites, making it possible to characterize their number and HX rates in both forms, and therefore the allosteric free-energy that they carry. (c) and (d) HX of functionally labeled Hb (35 min, 0 °C exchange-in) measured by the proteolytic fragmentation method with peptide separation by HPLC alone [38]. Two sets of allosterically sensitive amides are located at the segment level. They exchange by way of a local unfolding mechanism. (e) and (f) The integration of these approaches with mass spectrometric analysis which more closely defines some allosterically sensitive positions. (e) and (f) include MS data obtained with the same exchange-in as for the tritium experiments (open triangles), and more intensively (20 min, 20 °C, closed triangles) [35].