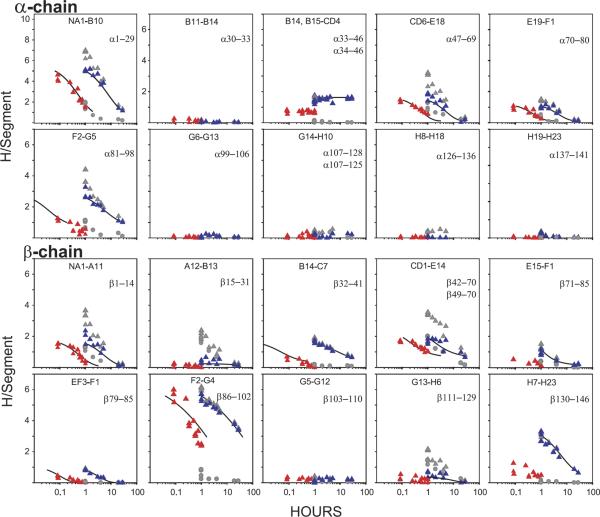
Figure 5.
H-D exchange/MS results for allosteric structure change in hemoglobin obtained as in Figure 4. Some allosterically sensitive amide sites were initially selectively labeled by the functional labeling method. Exchange-out results are compared for R-state oxy (red) and T-state deoxy (blue) Hb (obtained by subtracting the background from the raw data, both shown in gray; experimental details in [35]). HX was measured by the proteolytic fragmentation-separation method with analysis by MS. The peptides indicated span the entire length of the α- and the β-chains. The results show where changes occur and where they do not, and evaluate the number of amide sites affected within each sensitive segment. Several of the measured changes strongly suggest multi-residue segmental unfolding reactions (local unfolding) in which case the change in structural free-energy deposited at each allosterically sensitive position can be read out in terms of the change in HX rate between the two different allosteric forms (eq 3).