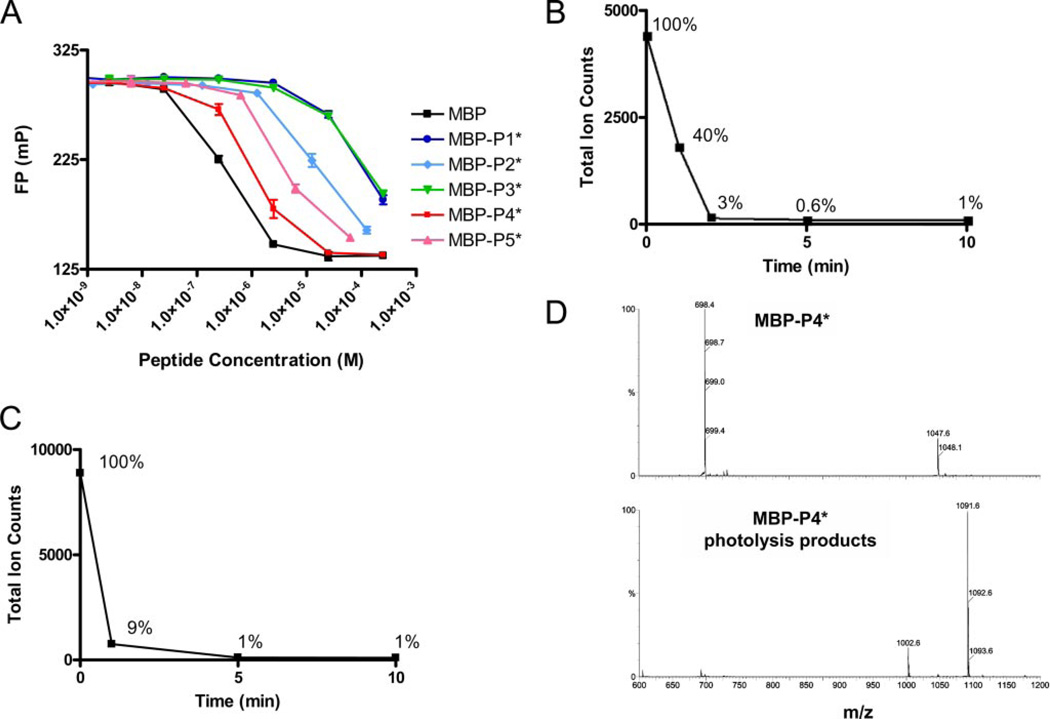
FIGURE 2. Affinity of photocleavable peptides for DR2 and kinetics of MBP-P4* cleavage.
A, photocleavable peptides MBP-P1* to MBP-P5* were examined for their ability to compete with MBP-488 (10 nm) for DR2 binding (100 nm). MBP-P4* bound most tightly. B and C, photocleavage of MBP-P4* was rapid both in solution or bound to DR2. D, mass spectrometry of intact peptide (top panel, 2+ and 3+ ions) and photocleaved peptide (bottom panel, 1+ ions) showing major cleavage products of molecular weights 1002.6 (C-terminal fragment) and 1091.6 (N-terminal fragment).