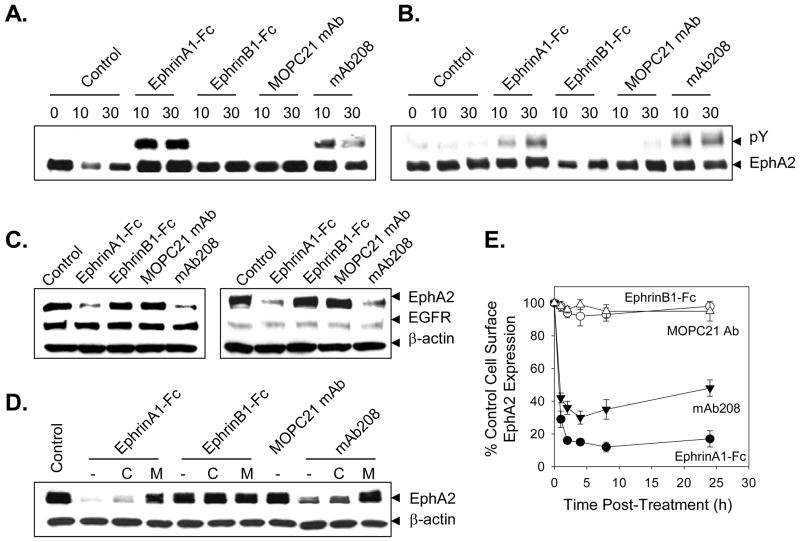
Figure 1. EphA2 agonists induce the phosphorylation and proteasome-dependent degradation of EphA2 in tumor cells in vitro.
In order to determine whether agonist treatment promotes EphA2 phosphorylation, SLR20.A2 (panel A) and SLR24 (panel B) renal carcinoma cells (2–4 × 106) were left untreated or were treated for 10m or 30m with EphrinA1-Fc, EphrinB1-Fc, MOPC21 mAb or mAb208 (each at 10 μg/ml) at 37°C. Cellular lysates were resolved by SDS-PAGE and EphA2 protein was immunoprecipitated using the anti-EphA2 antibodies D7 in pull-down assays. Western blot analyses were then performed using anti-EphA2 and anti-phosphotyrosine antibodies, respectively. To determine whether agonist treatment induces EphA2-specific degradation, SLR20.A2 (panel C) and SLR24 (panel D) tumor cells were treated as in panel A above for 24h hours with consequent cell lysates resolved by SDS-PAGE and Western blot analyses performed using polyclonal anti-EphA2 and control anti-β-actin antibodies. Anti-EGFR antibody was used to image identically-prepared lysates as a specificity (negative) control in these experiments. To assess the proteasome-dependence of agonist-induced EphA2 degradation, MG-132 (50 μM) or chloroquine (Chl.; 100 μM) were also added to SLR20.A2 cell cultures, where indicated, 30m prior to the addition of Ephrin-Fc proteins or mAb208 (panel E). After 24 h, cell lysates were generated and resolved using SDS-PAGE. Western blot analyses were then performed using anti-EphA2 antibodies and negative control anti-β-actin antibodies. In panel F, the kinetics of EphA2 down-modulation on the surface of treated (with the indicated agents) SLR20.A2 cells was investigated by flow cytometry. Data are reported as % control EphA2 cell surface expression (vs. untreated cells) based on mean fluorescence intensity values obtained. All data are representative of 3 independent experiments performed.