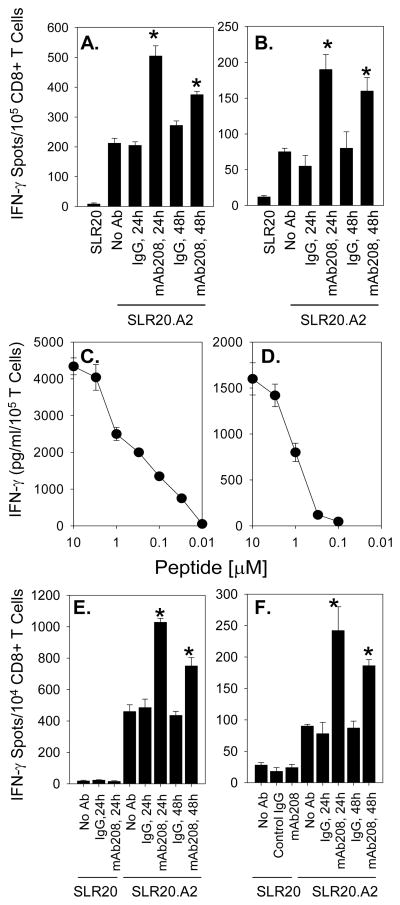
Figure 2. Anti-EphA2 mAb208 sensitizes the EphA2+, HLA-A2+ tumor cell line SLR20.A2 to recognition by anti-EphA2 CD8+ T cells in vitro.
Bulk CD8+ T cell lines were developed from HLA-A2+ normal donors against the EphA258-66 (panel A) and EphA2883-891 (panel B) peptides, as described in the Materials and Methods, and evaluated for their differential recognition of SLR20 (EphA2+, HLA-A2−) and SLR20.A2 (EphA2+, HLA-A2+) tumor cell targets using IFN-γ ELISPOT assays. SLR20.A2 cells were pre-treated with no Ab, control IgG (MOPC21 mAb) or mAb208 (10 μg/ml each) for 24h or 48h, as indicated, prior to ELISPOT analyses. Data are reported as mean IFN-γ specific spots/105 CD8+ T cells +/− SD from triplicate determinations. The HLA-A2 restricted CD8+ T cell clones 15/9 (specific for EphA258-66; panel C) and 3C1 (specific for EphA2883-891; panel D) were developed, as described in the Materials and Methods, and exhibited moderate-to-low functional avidity against specific peptide-pulsed T2 cells (EC50 approximately 1 μM peptide for both clones) as assessed by IFN-γ ELISA (mean +/− SD from triplicate determinations). These clones recognize SLR20.A2 tumor cells selectively in IFN-γ ELISPOT assays, with recognition increased at 24h and 48h by pretreatment of tumor cells with mAb208, but not control IgG. Data are reported as mean IFN-γ specific spots/104 CD8+ 15/9 (panel E) and 3C1 (panel F) T cells +/− SD from triplicate determinations. All data are representative of 3 independent experiments performed. *p < 0.05 vs. control IgG-treated tumor cells.