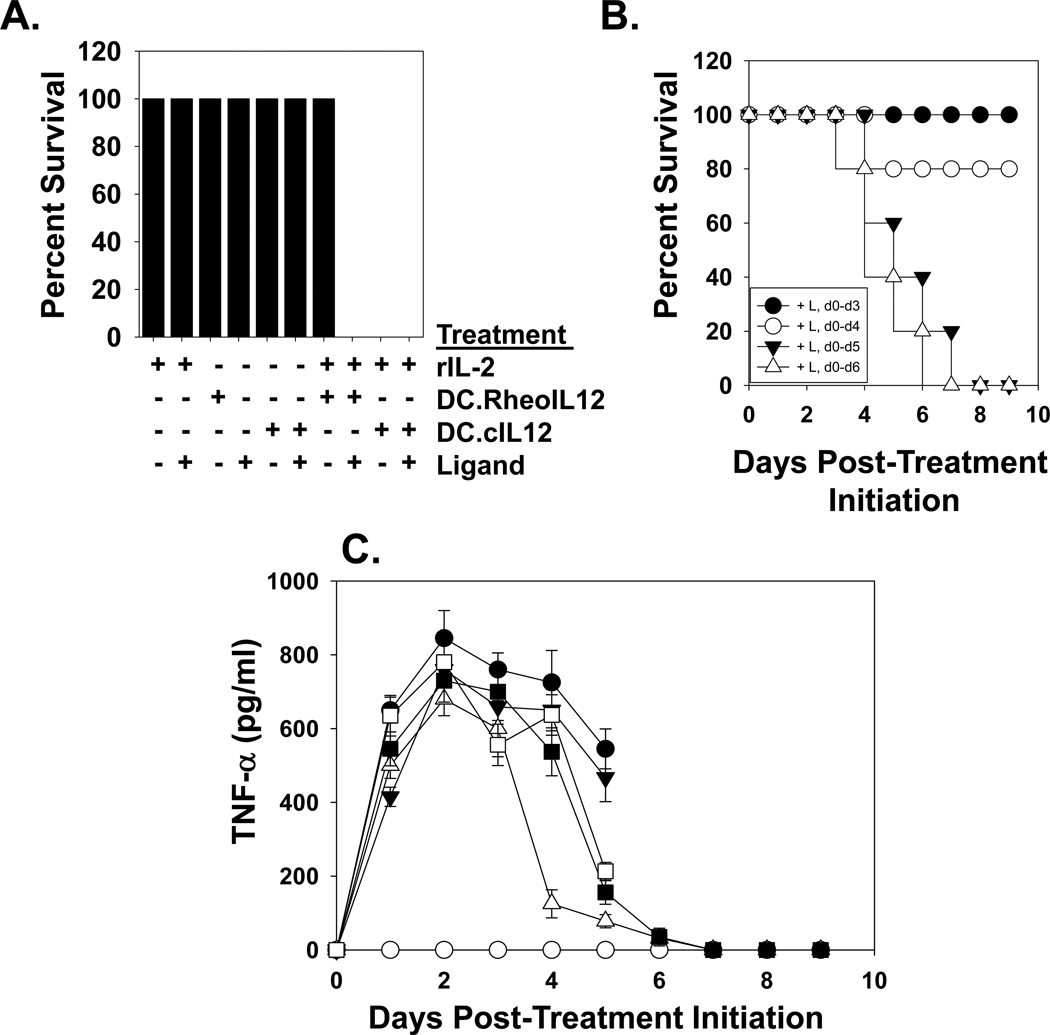
Figure 6. Discontinuation of activating ligand administration ameliorates toxicity associated with DC.RheoIL12 application in an IL-2 + IL-12 co-treatment model.
B6 mice were treated with i.p. injections of PBS, DC.RheoIL12 (+/− rIL-2 +/− activating ligand) or DC.cIL12, as outlined in the Materials and Methods. In panel A., no deaths were observed in cohorts receiving rhIL-2 only (+/− activating ligand for days 0–7), DC.RheoIL12 (+/− activating ligand for days 0–7), DC.cIL12 only (+/− activating ligand for 7 days) or rIL-2 + DC.RheoIL12 (in the absence of activating ligand). However, mice receiving either rIL-2 + DC.cIL12 or rIL-2 + DC.RheoIL12 + activating ligand for days 0–6 displayed severe toxicity. Data are reported on day 8 of the study. In panel B., discontinuation of activating ligand prevents the death of mice receiving IL-2 + DC.RheoIL12 injections. Activating ligand was provided during the indicated time intervals. In panel C., TNF-α was analyzed in serum obtained from the indicated animals by ELISA. Symbols represent mice treated with rhIL-2 plus: DC.cIL12 (●), DC.RheoIL12 without ligand (○); DC.RheoIL12 + ligand for days 0–3 only (Δ); DC.RheoIL12 + ligand for days 0–4 only (■); DC.RheoIL12 + ligand for days 0–5 only (□); DC.RheoIL12 + ligand on all days (▼). Data represent the mean +/− SD of data determined from triplicate ELISA determinations using all viable animals/cohort on a given day of analysis. For all panels, the reported data are representative of 2 independent experiments performed.