Abstract
Dendritic cells (DCs) are potent antigen-presenting cells responsible for the activation and functional polarization of specific T cells. In patients with renal cell carcinoma (RCC) and other cancers, coordinate DC and T cell defects have been reported. In particular, DC and T cell functional subsets that are not conducive to tumor clearance are hypothesized to predominate in patients with advanced-stage disease. Two major peripheral blood DC subsets have been identified in humans: myeloid dendritic cells (mDCs) and plasmacytoid dendritic cells (pDCs) that are believed to mediate contrasting effects on cancer immunity.
Given the lack of information regarding DC subsets in patients with RCC, in the present study we have investigated the comparative frequencies and activation states of mDC and pDC in peripheral blood, cancer tissues and lymph nodes of patients with RCC using flow cytometry and immunohistochemistry. Three monoclonal antibodies (mAbs) reactive against specific DC subsets (BDCA-2 or BDCA-4 for pDC and BDCA-1 and BDCA-3 which represent two distinct subsets of mDC, mDC1 and mDC2, respectively) were employed. We observed a significant reduction of both DC subsets in the peripheral blood of patients as compared to normal donors. Similarly, both mDC and pDC were recruited in large numbers into RCC tumor tissues, where they displayed an immature phenotype (DC-LAMP−) and appeared unable to differentiate into mature DC (CD83+) that were competent to migrate to draining lymph nodes.
However, we were readily able to generate ex vivo mDC from RCC patients. These DC stimulated robust anti-tumor CTL in vitro and would be envisioned for use in DC-based vaccines applied in patients with RCC whose existing immune system is judged dysfunctional, anergic or prone to undergo apoptosis.
Keywords: Renal cell carcinoma, dendritic cells, lymph nodes, confocal microscopy, T cell response
1.Introduction
Patients with advanced renal cell carcinoma (RCC) frequently exhibit immune dysfunction. Notably, patients with active, disseminated disease are typically characterized by predominant Th2-or T regulatory-type immunity (Tatsumi et al., 2002; Tatsumi et al., 2003). While RCC lesions are commonly observed to contain tumor-infiltrating lymphocytes, these cells are typically reported to be “functionally-inappropriate”, dysfunctional or pro-apoptotic, which is consistent with their inability to mediate clinically-beneficial outcomes in vivo (Van de Hove et al., 1997; Kolbeck et al, 1992).
Dendritic cells (DC) are the most potent antigen-presenting cells (APC) and continue to receive enormous attention as a “natural adjuvant” to be integrated in therapeutic vaccines in the cancer setting (Steinman, 1991). Induction of an effective anti-tumor response requires the active participation of DC, responsible for the capture of tumor-specific antigens (Ag) and transport of this “information” to regional lymphoid tissues, where tumor-specific T cells may be cross-primed. DC are heterogeneous in nature, being comprised of multiple cell subsets that display differences in terms of phenotype, functionality and tissue localization. Two major subsets of human blood DC have been defined (O’Doherty et al., 1994), and techniques for their isolation have been established (Dzionek et al., 2000). CD11c+/ CD123dim/ BDCA1+ DC, classically defined as “myeloid” DC (mDC), phagocytose antigens within their microenvironment and tend to be potent stimulators of Th1-type polarized T cell responses. In contrast, CD11c−/CD123hi/BDCA-2+ “plasmacytoid” DC (pDC) are poorly phagocytic when compared to mDC, and tend to support Th2-type immunity (Reid et al., 2000).
The presence of cancer leads to numerical and functional abnormalities of DC subsets in various tissues (blood, tumor and draining lymph nodes) that facilitate tumor-induced immune escape. Indeed, several reports have demonstrated that DC infiltrating a wide range of tumors have a deficient ability to stimulate anti-tumor T cell responses in vitro and in vivo (Perrot et al., 2007; Stoitzner et al., 2008). RCC tumors may prevent the induction of the immune response by releasing immunosuppressive factors including TGF-β, IL-10, gangliosides, products of oxidative stress and thrombospondin that inhibit immune responses by co-ordinately dampening down both DC and T cell function (Curiel et al., 2004; Kudo et al., 2003; Lusini et al., 2001). This microenvironment limits the degree of DC maturation and trafficking to lymph nodes, a process that is normally associated with prolonged survival and a reduced incidence of metastases in patients with various human cancers including melanoma, gastric, breast, oral, and lung carcinoma (Ladànyi et al., 2007; Tabarkiewicz et al., 2008; Iwamoto et al., 2003; Reichert et al., 2001). In the light of their profound importance to anti-tumor immunity, surprisingly little is known about the frequency or function of DC in RCC patients.
Using a panel of antibodies recognizing DC subsets and maturation markers (Dzionek et al., 2000), the primary goal of this study was to determine the comparative frequencies of the two major DC subsets in the peripheral blood and tumor tissues of 30 RCC patients as compared to 40 healthy individuals. Tumor-draining lymph nodes (LN) were also evaluated for mDC versus pDC frequencies in 13 of these patients. We report that the frequency of both DC subsets was significantly lower in the peripheral blood of RCC patients than of healthy controls, and that both DC subsets could be imaged in large numbers within RCC tissue, where they persist in an immature state that appeared incompetent to migrate into secondary lymphoid tissue. We also noted that peripheral, dysfunctional APC isolated from patients (Jonuleit et al., 1997) could be rendered immunostimulatory after in vitro culture in the presence of IL-2 and IL-7. These ex vivo generated mDC promoted superior CTL activity when loaded with RCC lysate as a source of tumor antigens and may constitute a novel vaccine component for patients with RCC.
2. Materials and methods
2.1. Antibodies
The mouse mAb anti-CD1c (BDCA-1; Miltenyi Biotec, Bergish Gladbach, Germany) recognizes a major subpopulation of human mDC (i.e. mDC1), while mouse mAb anti-BDCA-3 (Miltenyi) recognizes a minor subpopulation of human mDC (i.e. mDC2). The mouse mAb anti-CD11c (clone 5D11; Miltenyi) detects the human CD11c antigen on the membrane of histiocytes and DC (Novocastra). The mouse mAbs anti-BDCA-2 and anti-BDCA-4 (Miltenyi) recognize human pDC. As a marker of mature DC we used mAb DC-LAMP (clone 104.G4; Immunotech-France). The mouse mAb anti-CD83 antibody (clone 1H4b; Abcam) recognizes the human CD83 antigen of activated peripheral blood DCs and interdigitating reticulum cells within the T cell zones of lymphoid organs. The following FITC- or PE-conjugated mAbs were also used for immunofluorescent staining of mDCs: FITC-HLADR, PE-CD11c, PE-CD86, FITC-CD80, FITC-CD40, PE-CD54, PE-CD83, isotype control (all BD-Pharmigen) PE-CCR7 (R&D Systems). For the surface staining of T cells, three-color fluorescence conjugated mAbs were used: APC-CD8, PE-CD4, FITC-CD45RA, FITC-CD62L, FITC-CD45RO, FITC-CD28, FITC-CCR7 (R&D Systems), FITC-CD27 (e-Biosciences).
2.2. Enumeration of circulating DCs in RCC patients
Flow-cytometric analysis was used to analyze DC subsets in patient peripheral blood in concert with a Blood Dendritic Cell Enumeration Kit (Miltenyi). Briefly, the scatter properties of the cells, as well as staining with CD19 and CD14 monoclonal antibodies (mAbs), enabled enumeration of B cells and monocytes, respectively. Simultaneous labeling with anti-CD1c (BDCA-1) and anti–BDCA-2 mAbs allowed identification of CD19 cells and enumeration of the BDCA-1+ mDC and the BDCA-2+ pDC populations. Absolute numbers of the respective cell populations were normalized to the total number of PBMCs collected, yielding the relative frequencies of each cell type.
2.3. Renal Tissue Biopsies
RCC tissue samples were obtained from patients with a CT-confirmed renal mass and signed consent under an institutional review board approved protocol. The main demographic and clinical features of the patients population are summarized in table 1. At the time of surgery, all the patients showed no evidence of disease (NED) except one. Clinical and pathological findings was gathered for staging at operation. T stage was defined by pathological examination and the N and M components was defined according to pathological findings or by clinical data when applicable. Pathological stage of the primary tumor (pT), regional lymph node status (N) and metastases were assigned using the 2002 version of the TNM staging system (Greene FL, et al: AJCC Cancer Staging Manual, 6th ed. New York: Springer-Verlag 2002). Heidelberg classification (Kovacs et al., 1997) and Fuhrman grading system (Patard et al., 2003) were used to assign histotype and grade of differentiation, respectively. Tumors, normal-appearing kidney portions and lymph nodes (when available) were removed aseptically during surgery: radical nephrectomy including standard loco-regional lymph node dissection and partial nephrectomy or tumor enucleation with no lymph node removal, by open or videolaparoscopic procedures, were performed. Lymph nodes from patients not affected by renal diseases were used as controls. Removed tissues were immediately embedded in OCT compound, snap-frozen and stored in liquid nitrogen until use. Portions of tissue samples were fixed in 10% buffered formalin and paraffin-embedded. Inclusion criteria were histologically confirmed RCC, of the clear cell subtype, not previously submitted to pre-operative therapy. Tissues derived from biopsies of patients with microhematuria and no histological abnormalities were also collected and used as normal controls. Tissue samples were processed for crude lysate preparation by manual disruption and enzymatic digestion, centrifuged, aliquoted and stored at −80°c until use. The data about RCC patient follow-up were collected according to the European Association of Urology guidelines (EAU 2007), consisting mainly in six month visit, physical examination, chest X-rays, abdomen imaging, serum assessment (creatinine, haemoglobin, alkaline phosphatase).
Table I.
RCC patients evaluated in the study, considering age at time of surgery, sex, pathological stage, grade (Fuhrman), adjuvant treatment and at the last evaluation, follow-up months.
| Patient | Age/Sex | Stage | Grade | Adjuvant Treatment |
Last Evaluation |
Follow-up (months) |
|
|---|---|---|---|---|---|---|---|
| DG | 63 | M | T3aN0M0 | 3 | - | NED | 55 |
| CG | 55 | M | T1N0M0 | 1 | - | NED | 67 |
| CA | 74 | M | T3aN0M0 | 2 | - | NED | 65 |
| SO | 46 | F | T3aN0M0 | 2 | - | NED | 52 |
| B M | 76 | F | T1N0M0 | 3 | - | NED | 39 |
| CG | 53 | F | T3aN0M0 | 3 | - | NED | 53 |
| LA | 67 | F | T3bN0M0 | 2 | - | NED | 58 |
| MR | 45 | M | T3aN0M0 | 3 | - | NED | 71 |
| ZG | 58 | M | T3aN0M0 | 3 | - | NED | 103 |
| RG | 66 | M | T3bN0M0 | 3 | - | Mets, liver-chest | 32 |
| SL | 49 | F | T3aN0M0 | 1 | - | NED | 60 |
| PT | 52 | F | T3aN0M0 | 2 | - | NED | 57 |
| CB | 82 | M | T1N0M0 | 1 | - | NED | 46 |
| RS | 45 | M | T2N0M0 | 2 | C, RT, S | Mets, bone | 22 |
| BA | 29 | M | T1N0M0 | 2 | - | NED | 67 |
| PG | 67 | F | T3aN0M0 | 2 | - | NED | 63 |
| CL | 77 | M | T1N0M0 | 2 | - | NED | 28 |
| CM | 78 | F | T3bN1M0 | 3 | - | Mets, lung-spleen | 62 |
| NC | 81 | M | T1N0M0 | 1 | - | NED | 74 |
| SL | 53 | M | T3aN0M0 | 3 | S, IL-2 | Mets, lung | 57 |
| GR | 69 | M | T3aN0M0 | 3 | C, IL-2 | Mets, lung | 41 |
| CA | 72 | M | T1N0M0 | 1 | - | NED | 50 |
| TC | 62 | M | T3aN0M0 | 1 | RT, S | Mets, bone | 54 |
| BM | 53 | M | T1N0M0 | 2 | - | NED | 36 |
| SV | 68 | M | T3aN0M0 | 1 | - | NED | 32 |
| DeG | 60 | M | T3bN0M1 | 2 | - | Mets, lung | 18 |
| PS | 76 | F | T3aN0M0 | 2 | - | NED | 18 |
| TN | 74 | F | T3aN0M0 | 2 | - | NED | 25 |
Abbreviations Used: C, Chemotherapy; Mets, Metastatic Disease; S, Surgery; IL-2, Interleukin 2 Therapy; RT: radiotherapy; NED, No evidence of Disease.
2.4. Tissue Immunofluorescence
The expressions of BDCA-1, BDCA-3, BDCA-4 three markers for distinct subsets of dendritic cells and DC-Lamp a specific marker of mature DC, were evaluated by indirect immunofluorescence and confocal analysis microscopy on renal frozen tissue. The fixed sections were washed in phosphate buffer saline (PBS) pH 7.4 and then incubated: (1) for 45 min in blocking buffer [PBS pH 7.4, 10% Goat Serum (Sigma)] at room temperature in a dark and humidified container, (2) overnight at 4°C in primary antibody diluted in blocking buffer (the anti-BDCA-1 and anti-BDCA-3 antibodies diluted 1:20, the anti-BDCA-4 and anti-DC-LAMP antibodies diluted 1:100) and (3) for 2h with the appropriate secondary antibodies (Alexa Fluor 488 goat anti-mouse IgG, diluted 1:400, Molecular Probes) at room temperature. The sections were washed in PBS after each step, counterstained with TO-PRO-3 (Molecular Probes), mounted in GEL/MOUNT (Biomeda Corp., Foster City, CA/USA) and finally sealed with nail varnish. Negative controls were prepared by omitting the primary antibodies. The expression of CD11c, a marker of myeloid-derived DC, and CD83, a specific marker of mature DC, were evaluated by indirect double-immunofluorescence and confocal microscopy analysis on 6 micron sections produced from paraffin-embedded human lymph node.
Sections were incubated for 1h in blocking buffer (2% bovine serum albumin in PBS; Invitrogen) and then with mAb (1:40 dilution) anti-CD83 and anti-CD-11c (1:100 dilution) for 1h at room temperature and overnight at 4°C, respectively. Immune complexes were identified after incubation of the sections with the following secondary antibodies: Alexa Fluor 488 goat anti-mouse IgG2a and 555-labeled goat anti-mouse IgG1 (Molecular Probes, Eugene, OR) (1:200 dilution) for 1h at room temperature. The sections were washed in PBS 1X after each step, counterstained with TO-PRO-3 (Molecular Probes). The slides were then mounted on Gel/Mount (Biomeda, Milan, Italy) and sealed.
2.5. Confocal Laser Scanning Microscopy
Confocal microscopy was performed using a Leica TCS SP2 (Leica, Wetzlar, Germany) microscope equipped with argon-krypton (488 nm), green-neon (543 nm) and helium-neon (633 nm) lasers. Confocal images were taken at 500-nm intervals on z-axis of the section covering a total 6 micron depth. Images from individual optical planes and multiple serial optical sections were analyzed and the images were sequentially scanned at the 3 wavelengths. The number of BDCA-1+, BDCA-3+, BDCA-4+, DC-LAMP+ and CD11c+/CD83+ cells was measured in at least fifteen high power fields (HPF; × 630)/section by two independent observers blinded to the origin of the slides. The final count was the mean of the two measures. In no case the inter-observer variability exceed 20%.
2.6. Isolation of PBMC and preparation of mDC
Peripheral blood was obtained by venipuncture from 6 RCC patients with signed consent and peripheral blood mononuclear cells (PBMC) were isolated at the interface of ficoll-hypaque density gradient (Sigma Chemical Co.; St. Louis, MO) per the manufacturer’s instructions, washed twice in PBS, re-suspended in AIM-V medium (Invitrogen-Life Technologies; Carlsbad, CA) and incubated at 37°C for 2h to allow the adhesion of monocytes to plastic. Adherent monocytes were cultured with 1000U/ml GM-CSF and 1000 U/ml IL-4 (Schering-Plough) in AIM-V medium. DC were pulsed on day 5 with an average of 100 µg/ml tumor lysate at 37°C overnight. Tumor cells lysate was obtained by 5 freeze-thaw cycles (liquid nitrogen freezing followed by thawing at 37°C × 5 cycles). Supernatants were passed through a 0.45-µm filter. Protein content was determined using a Bradford protein assay (Bio-Rad) and aliquots were stored at −80°C until use. On day 6, to generate mature DC, PGE2 (10−6 mol/L), TNF-α (50 ng/mL; Strathman Biotech), IL-1β (25 ng/mL; Stathmann Biotech, Hamburg, Germany) and IL-6 (1000 U/mL; PeproTech) were added to culture. On day 8 of culture, mDC were harvested and analyzed by flow cytometry, then tested for their ability to stimulate RCC specific T cell responses in vitro.
2.7. Immunophenotypic analyses
Cells were washed and resuspended in FACS buffer (PBS pH 7.2, 0.2% BSA, and 0.02 % sodium azide) containing 3% human serum and incubated with fluorochrome-conjugated mAbs for 15 min at 4°C, then washed with the same buffer prior to flow cytometric analysis. Data were acquired using a FACSortTM (Becton Dickinson) flow cytometer and analyzed using WinMDI Version 2.8 software.
2.8. In vitro stimulation (IVS) of T cells
Previously harvested peripheral blood lymphocytes (PBL), were cultured at 1–2 × 106 per well in 24 well plates containing RPMI/10% heat-inactivated pooled human serum [Sigma (medium Mb)] along with autologous DC pulsed with renal tumor lysate (at a 10:1 T cell:DC ratio), in the presence of 1 ng/ml IL-12p70 (R&D Systems, Minneapolis, MN) and 1000 UI/ml IL-6 (PeproTech). Responder lymphocytes were re-stimulated twice on a weekly schedule with mature DC in RPMI/10% human serum AB supplemented with IL-2 (20 units/mL; Proleukin; Novartis) and IL-7 (5ng/mL; R & D Systems, Minneapolis, MN).
2.9. ELISPOT Assays
On day 21 of the IVS cultures, T cells were assessed for their effector cytokine profiles using h-IFN-γ ELISPOT assays (BD-PharMingen; San Diego, CA), as recently described (Tatsumi et al., 2002). T cells were added to ELISPOT wells along with tumor lysate-loaded DC at a 10:1 T cell:DC ratio and plates were subsequently incubated at 37°C for 24 hours to detect IFN-γ producing cells. Determinations were performed in triplicate and spots were enumerated using an automatic plate reader (Zeiss-Kontron, Jena, Germany).
2.10. Statistical Considerations
All data are expressed as means ± SD. Statistical differences between groups were evaluated using 2-tailed Student’s t test, and P values <0.05 were considered significant.
3. Results
3.1. Assessment of the frequency of DC subsets in the peripheral blood of RCC patients
We investigated the comparative frequencies of mDCs and pDCs in a group of 30 RCC patients and 40 healthy donors (MacDonald et al., 2002). Table 1 summarizes two patient cohorts assessed: patients with documented metastases at their last clinic evaluation (n=7) and patients with no evidence of disease (n=23).
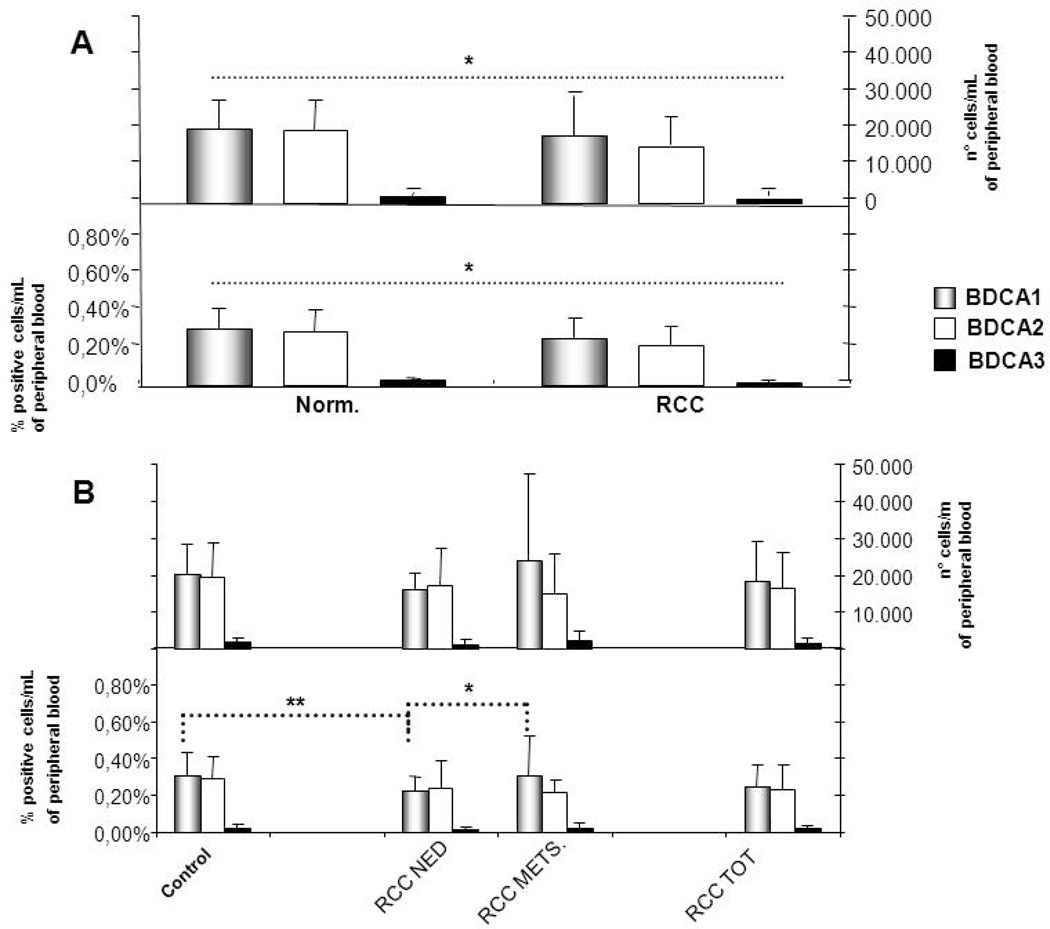
We identified two subpopulations of circulating blood mDCs (i.e. mDC1 are BDCA-1+; mDC2 are BDCA-3+) and pDCs as BDCA-2+ cells. As shown in Fig.1A, among the RCC patients the frequency and the absolute number of both DC subpopulations (DC total) were significantly reduced when compared with normal donor controls (P<0.01). The obtained frequencies of mDC1, mDC2 and pDC were 0.23 ± 0.08%, 0.22 ± 0.11% and 0.01 ± 0.01%, respectively, while the absolute numbers of cells were 15949 ± 4558, 14836 ± 7304 and 864 ± 564, respectively. In the control group, the percentages of mDC1, mDC2 and pDC were 0.30 ± 0.12%, 0.29 ± 0.12% and 0.03 ± 0.01% respectively and the absolute numbers of cells were 20393 ±7730, 19888 ± 8419 and 1897 ± 866, respectively. As shown in Fig. 1B, the median frequency of mDC1 was significantly increased in metastatic RCC patients (0.31%) when compared with patients with no evidence of disease or normal donor controls (p < 0.05). A reduced frequency of mDC1 was observed in RCC patients with no evidence of disease vs. normal donor controls (p < 0.05). Interestingly, we did not find a reduction of the circulating levels of total leukocytes in patients, suggesting that the decrease in DC frequencies was not correlated with a variation in peripheral blood monuclear cells number (data not shown).
Figure 1. Circulating mDC and pDC in RCC patients.
(A). Blood samples were obtained from 30 RCC patients and 40 healthy volunteers. Four-color flow cytometry was used to distinguish mDC and pDC subsets within freshly-isolated peripheral blood mononuclear cells using specific antibodies directed against mDC1 (anti-BDCA1), mDC2 (anti-BDCA3), and pDC (anti-BDCA2). * P< 0.01 for all tested subpopulations. (B). The frequency of DC subsets in metastatic RCC patients (METS) vs. patients with no evidence of disease (NED) at last clinic evaluation of disease. * P<0.05; ** P<0.01.
3.2. In situ characterization of DC in RCC tumors and tumor-draining lymph nodes
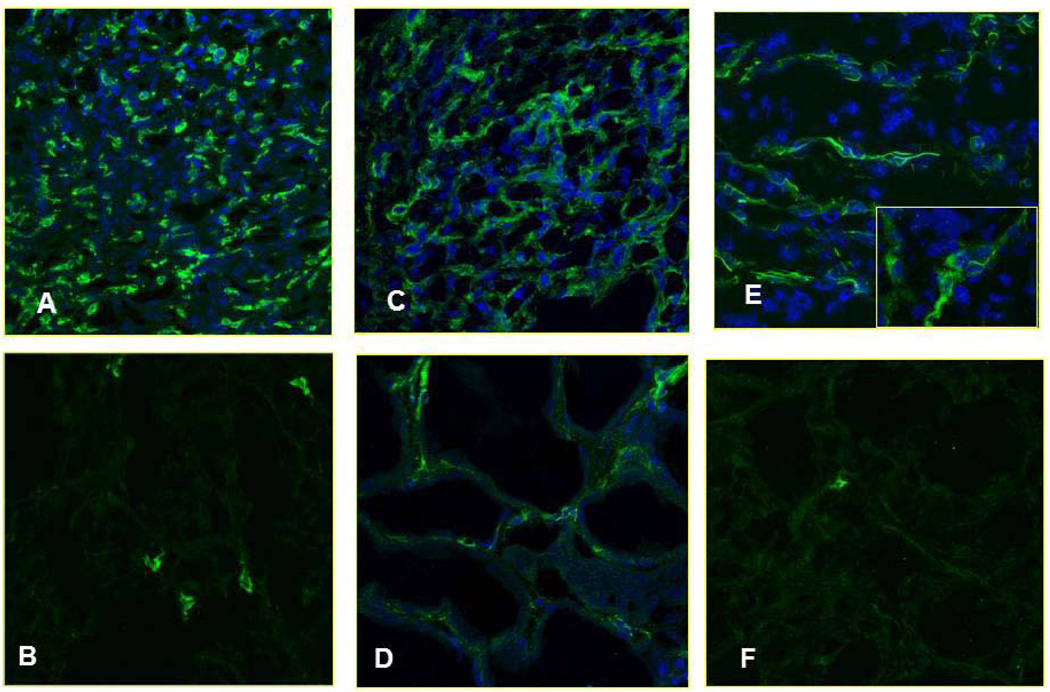
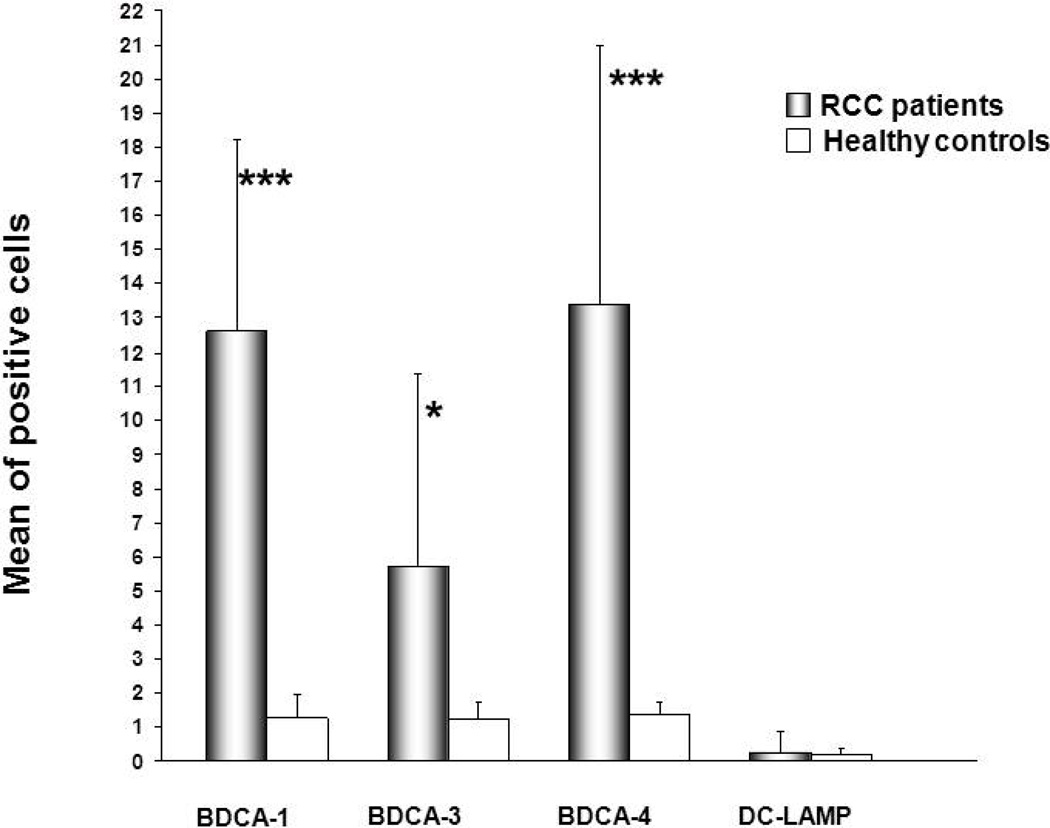
The low proportion of DC in the peripheral blood of RCC patients could be explained by at least two potential mechanisms: i.) preferential attraction of DC into patient tissues, or ii.) defective development of DC from bone marrow precursors. To evaluate these possibilities, we analyzed renal biopsies isolated from RCC and normal patients for the presence of mDC (Dzionek et al., 2000). Indirect immunofluorescence and confocal microscopy analyses revealed a high number of infiltrating immature myeloid DC to be present within the interstitial areas of renal biopsies obtained from RCC patients when compared to normal kidney. Interestingly, the number of infiltrating BDCA1+ mDC1 was most clearly increased in RCC patients, suggesting its likely efficient recruitment from the peripheral blood (Fig. 2A, B). The pattern for BDCA-3 staining resembled that for BDCA-1 (Fig. 2E, F). Interestingly, BDCA3+ mDC2 exhibited longer dendritic processes surrounding kidney tubular epithelial cells when compared to BDCA1+ DC1 (Fig. 2E, zoom). Overall, the mDC1/mDC2 frequencies in RCC tissues were significantly increased (BDCA-1+ cells: p=0.01; BDCA-3+ cells: p=0.04) when compared to normal kidney tissue (Fig. 4).
Figure 2. Analysis of tumor-infiltrating mDC and pDC in RCC kidney using confocal laser scanning microscopy.
Immunofluorescent indirect staining of mDCs was performed by using an anti-BDCA1 Ab in RCC and healthy control respectively (A and B), or anti-BDCA3 Ab in RCC and healthy control respectively (E and F) and staining of pDC by using an anti-BDCA4 Ab in RCC and healthy control respectively (C and D). Nuclei are highlighted with TO-PRO in blue, (magnification 40× for A, B, C and F, 63× for D and E).
Fig. 4. Summary of DC subset quantitaion in tissues.
Quantitative analysis of mean fluorescence intensity (MFI) was carried out by confocal microscopy software (Leica, TCS-SP2). Results are expressed as mean ±SD of BDCA1, BDCA3, BDCA4, DC-LAMP in RCC patients versus healthy controls (*** P< 0.01, P< 0.05).
3.3. Analysis of BDCA-4 expression in RCC vs. normal kidney tissue
We next investigated RCC versus normal kidney tissues for expression of BDCA-4, an antigen that is highly restricted to pDC in both the blood and peripheral tissues (Tatsumi et al., 2002; Tatsumi et al., 2003). Immunofluorescence analyses of frozen tissues showed that under normal conditions, pDCs are very rare in peripheral tissues, as they can migrate directly from blood to lymphoid tissues via high endothelial venules (Cella et al., 1999). On the contrary, we observed massive infiltration of RCC tissues by BDCA4+ pDC in all tubulointerstitial areas (Fig. 2C, D). When compared with normal kidney tissues, this difference in pDC infiltration was highly-significant (p = 0.01; Fig. 4).
3.4. Analysis of the state of DC maturation in RCC vs. normal kidney tissue
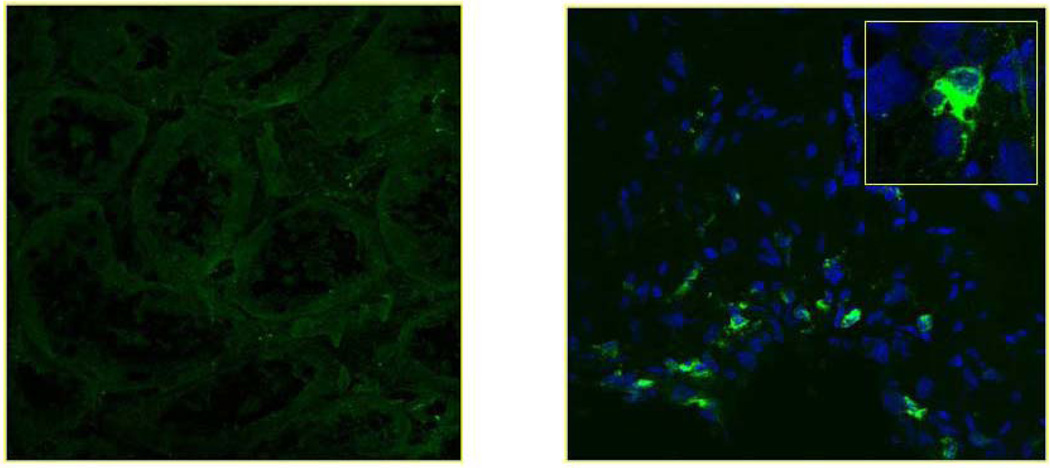
To investigate the relative state of activation for kidney infiltrating DCs, we analyzed these cells for their expression of DC-LAMP, a protein belonging to the family of lysosomal-associated glycoproteins and considered to represent a highly-specific marker of human DC maturation (Saint-Vis et al., 1998). Indirect immunofluorescence and confocal microscopy analyses suggest that DC-LAMP+ cells were rare events in RCC tissue, with the few positive cells being randomly distributed within the interstitial area of the kidney (Fig. 3A, B, respectively). This data is consistent with that obtained for other peripheral tissues, with infiltrating DC bearing an immature phenotype. We noted no significant difference in the frequency of DC-LAMP+ frequencies in RCC vs. normal kidney tissue (p = 0.2; Fig. 4).
Fig.3. In situ analysis for DC-LAMP+ DC in RCC tissues.
(A, B). Confocal laser scanning microscopy of DC-LAMP expression on mature DC in healthy control and RCC tissues, respectively (magnification 40×).
3.5. Analysis of DC in the tumor-draining lymph nodes of RCC patients
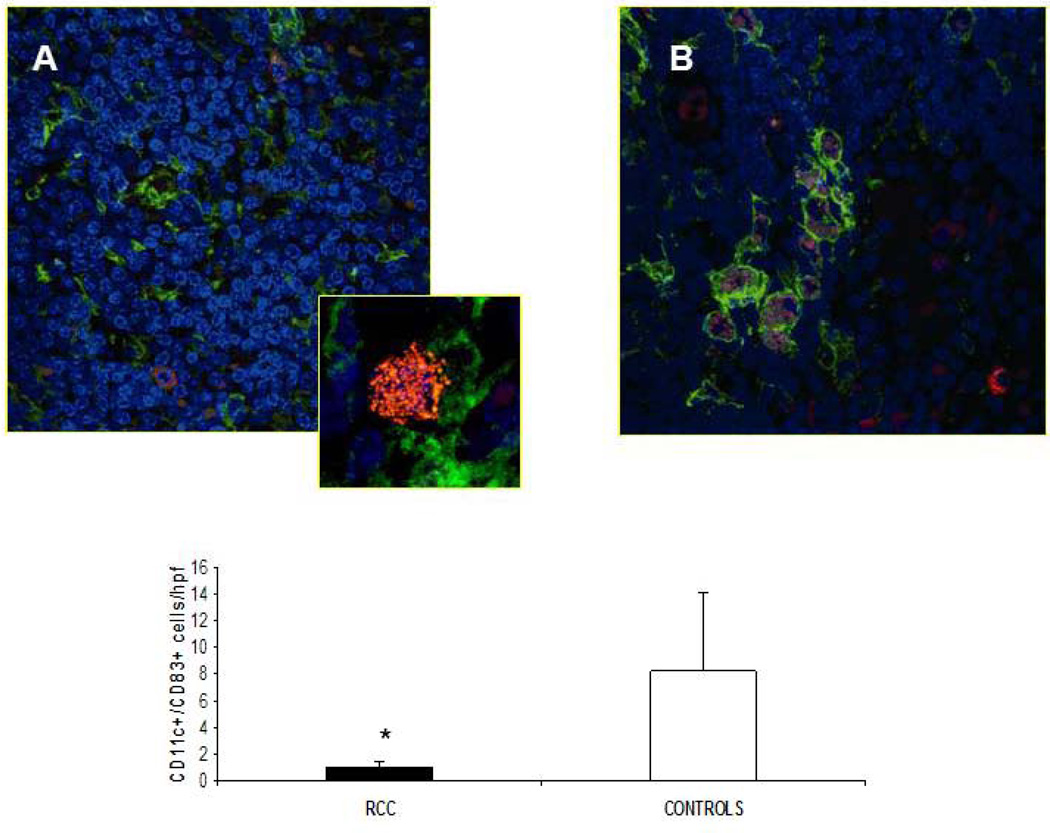
In response to pathogens or inflammatory stimuli, peripheral DC undergo phenotypic and functional maturation, leading to their migration via the afferent lymphatic system to the T-cell-rich areas of lymph nodes. After their arrival, they encounter naive T-cells and initiate adaptive immune responses (von Andrian, Mempel, 2003). There is accumulating evidence that the LN represents a primary basin of tumor-induced DC/T cell dysfunction (Poindexter et al., 2004; Lee et al., 2005). To elucidate DC frequencies within LN and determine whether they displayed altered phenotypes, lymph node sections were analyzed by immunofluorescence (confocal) microscopy using mAbs directed against CD11c (myeloid DC) and CD83 (mature DC; Lechmann et al., 2001). LN harvested from a total of 12 RCC patients and 4 normal subjects were inspected. RCC-LN were generally characterized by high-frequencies of CD11c+ DCs, most of which were of a CD11c+/CD83− phenotype (Fig. 5A, B). Frequencies of CD11c/CD83 dual-positive cells were statistically different when comparing RCC-LN versus lymph nodes harvested from normal donors (P = 0.01). These data are in accordance with previous reports in other human cancers (melanoma, breast and lung cancer), in which, the degree of infltration by mature DCs was inversely correlated with prolonged survival and a reduced incidence of metastases (Movassagh et al., 2004).
Fig. 5. Infiltrating CD11c+ CD83+ DCs in lymph nodes of RCC.
(A, B). The presence of mature DC was analyzed by confocal microscopy using antibodies that recognize mDC (anti-CD11c, green) and mature DC (anti-CD83, red), in RCC patients and healthy controls respectively (magnification 63×). Panel A shows a morphological particular of CD11c+/CD83+ DC (zoom 2×) (C). Quantification of CD11c-CD83 double-positive T cells. Results are expressed as mean ± SD of CD11c+ CD83+ in RCC patients versus healthy controls. (* P < 0.03).
3.6. Functional assessment of ex vivo generated mDC1 to promote specific Type-1 T cell responses in RCC patients in vitro
Given the comparative reduction in circulating levels of mDC1 in RCC patients, that are believed to be required for optimal Type-1 anti-tumor immunity, we next chose to evaluate whether mDC1 could be generated ex vivo from peripheral blood precursors isolated from these patients. In particular, we wanted to assess whether such cultured mDC1 would be competent to promote the effective development of tumor-specific CTL. mDC1 were generated from 6 RCC patients, pulsed with autologous RCC lysate and then used as an in vitro stimulus for autologous peripheral blood T cells. mDC1 were generated according to standard procedure using rGM-CSF and rIL-4 supplementation for 6 days, followed by application of cytokine maturational-cocktail (i.e. IL-1β + TNFα + IL-6 + PGE2) for 2 additional days. These cells exhibited a fully mature surface phenotype (de Vries et al., 2003) based on high expression levels of the CD80, CD83, CD86 and CCR7 markers (data not shown).
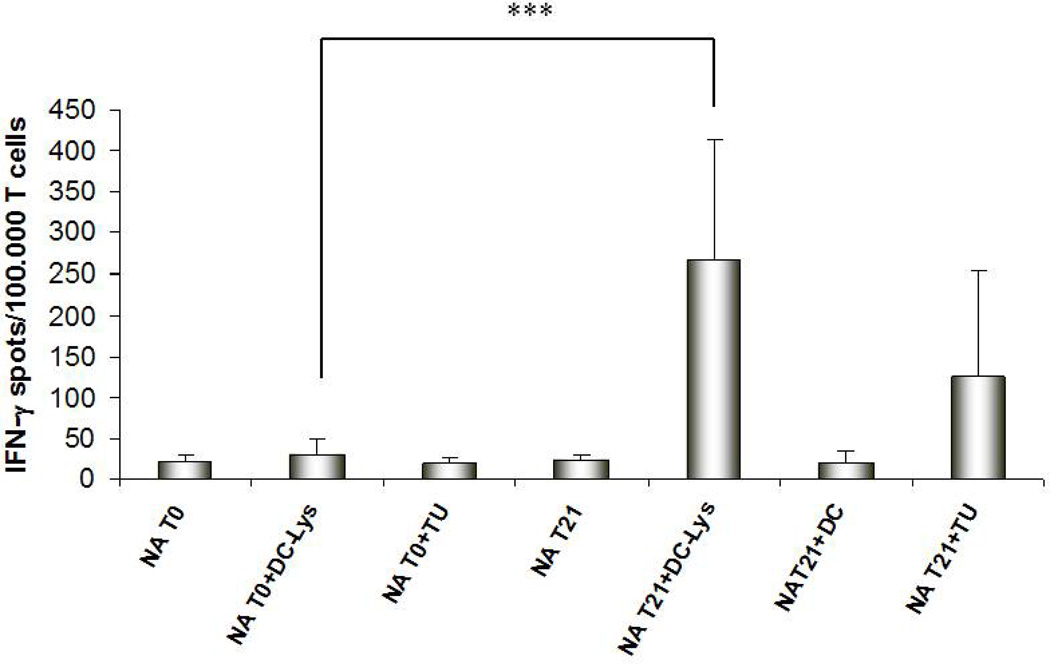
T cell lines obtained after multiple rounds of stimulation using antigen-loaded mDC1 were harvested at day 21 of culture and tested for Type-1 functional status using IFN-γ ELISPOT readout assays. We analyzed the specificity of CTL responses under basal conditions (T0) and after 21 days of in vitro stimulation (T21). As shown in Figure 6, at T0, T cells displayed a low degree of reactivity against mDC pulsed with autologous tumor lysate. After in vitro stimulation (T21), there was a strong increase in IFN-γ-secreting T cells responding against the same stimulus (p < 0.001). T21 cells also exhibited significant, albeit lower level, responses against autologous tumor cells themselves.
Fig.6. IFN-γ ELISPOT analyses of RCC-specific T-cell responses after mDC-based stimulation in vitro.
Frequencies of responder T cells reactive against RCC are reported for six RCC patients after in vitro stimulation with tumor lysate-loaded MoDC. Results represent the average (+/− SD) of triplicate wells of values obtained from the analysis of 6 RCC patients. *** P < 0.001.
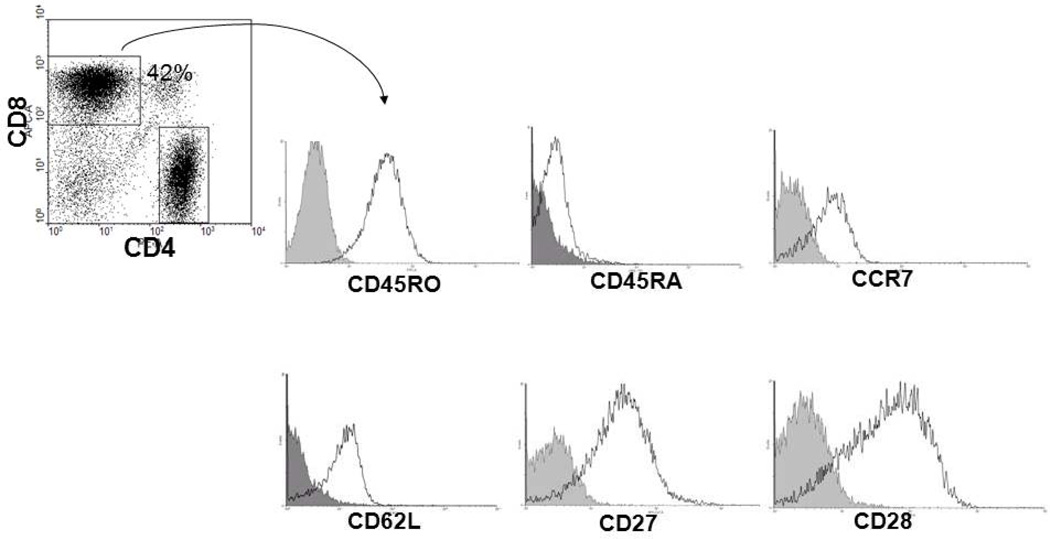
We further characterized the tumor-specific T cells generated using mDC1-based stimulations, focusing on markers of T cell activation, proliferation and differentiation (Fig. 7). Staining of isolated T cells after in vitro stimulation with lysate-loaded mDC1 showed that a majority of CD8+ T cells expressed a CD45RA− CD62+ CCR7+ phenotype, consistent with “central memory” T cells (Sallusto et al., 2004). Such T cells would be presumed to more readily respond to antigenic stimulation given their capacity to home to the lymph node, where they may be induced to expand and generate both memory and effector anti-tumor T cells. Importantly, these cells were also determined to express both CD27 and CD28, a phenotype that has been linked to long-term persistence after adoptive transfer into cancer patients (Powell et al., 2005; Ochsenbein et al., 2004). When taken collectively, these results suggest that: i.) mDC1 can be reproducibly generated from the blood of RCC patients and ii.) mDC1 can effectively promote the in vitro activation and expansion of Type-1 anti-tumor T cells (that might be applied in adoptive immunotherapy approaches for these patients).
Fig.7. Phenotypes of memory/effector responder T cells.
Expression of the CD45RO, CD62L, CCR7, CD27 and CD28 cell surface molecules was analyzed on CD8+ T cells after DC-based in vitro stimulation. Data shown are gated on CD8+ T cells and quadrants were established based on T cell staining with isotype control mAbs. Results are depicted for one patient RCC, but are representative of the 6 RCC patients evaluated.
4. Discussion and conclusion
In the present study, using a panel of antibodies recognizing DC subsets and their maturation markers, we investigated the presence of both mDC and pDC subpopulations in the peripheral blood, tumour tissue and lymph nodes of RCC patients. We found a significantly lower percentage of both types of DC in the peripheral blood of RCC patients when compared with healthy donors. Clearly, such reductions in systemic DC frequencies could be related to the inhibitory effects of tumor-elaborated factors, such as IL-6, IL-10, VEGF and/or gangliosides (among others) that block DC maturation or promote their apoptosis (Kudo et al., 2003; Lusini et al., 2001). Alternatively, lower levels of DC subsets in the blood could reflect the recruitment of DC into the tissues of RCC patients. Indeed, we noted a significantly higher number of infiltrating mDC (BDCA-1+ and BDCA-3+) and pDC (BDCA-4+) in RCC tissues when compared to normal donor kidney tissues. However, these cells did not express the maturation marker DC-LAMP that is considered prognostic of the ability of these APC to stimulate immune response associated with the extended survival of melanoma patients (Movassagh et al., 2004). Indeed, immature or incompletely-matured mDC, as well as, pDC may promote immune tolerance rather than immune activation against tumor-associated antigens (Steinman, 1991). They may also promote T-cell anergy or activate regulatory T cells (Curiel et al., 2004).
To determine whether tumor-associated alterations in the mDC and pDC numbers/phenotype extended to additional tissues, we analyzed the tumor-draining lymph nodes (LN) of 6 RCC patients, that is the first node accessed by cells leaving the tumor site via the lymphatic ducts and is a principal site in which anti-tumor T-cell priming may occur in cancer patients. Previous studies have demonstrated that the extent of mature DC trafficking to LN is correlated with local expansion of efficient antitumor T-cell-mediated immune responses and a favourable clinical outcome in melanoma patients (Ladànyi et al., 2007). Interestingly, we observed a significantly lower degree of mature myeloid-derived DC (CD11c+/CD83+) infiltration when compared to normal controls and it was in conflict with the physiological status where mature DC present tumor antigens to naïve T cells, resulting in T cell activation and differentiation. On the contrary, we found a high frequency of immature DC. The interference with maturation of myeloid-derived DC subset, which appears to be linked to the presence of tumor, could well contribute to immunosuppression of RCC patients and their failure to establish an effective antitumor immune response. Collectively, these data suggest that DC in RCC patients may be sequestered within tumor lesions where that exhibit reduced antigen-presenting function. Inefficient antigen presentation extends to the tumor-draining lymph node, thereby limiting the generation of protective anti-tumor immune responses in these individuals.
Despite the apparent disease-associated alterations in RCC patient DC subpopulations, we chose to next evaluate whether immunogenic DC could be developed from these individuals using an ex vivo culture approach. DC loaded with tumour antigens are considered an effective vaccine modality and has proven both safe and immunogenic in phase I/II clinical trials for a range of cancer types. Hence, the use of fully functional DC (i.e. DC1-type cells) in RCC immunotherapy would appear to represent a promising modality to elicit/expand or “reform” clinically-beneficial Type-1 anti-tumor immune responses. Such approaches are clearly warranted since RCC that has proven refractory to conventional treatment modalities, such as chemo- and radio-therapy. We observed that cultured patient DC exhibited a fully-mature surface phenotype expressing high levels of the maturation-associated markers CD54, CD80, CD40, CD86, CD83 and CCR7 and that these APC were capable of effectively promoting the in vitro expansion of RCC-specific, IFN-γ-producing T cells. Interestingly, we observed that in vitro stimulation of T cells with lysate-loaded MoDCs induced higher percentages of CD45RO+, CCR7+, CD62L+ T cells, consistent with the “central memory” T cell phenotype associated with superior therapeutic potential in adoptive therapy applications (Klebanoff et al., 2005; Gattinoni et al., 2005). In this regard, the likely importance of DC1-based immunotherapy in RCC patients has been recently established in our previous study, where we demonstrated that mDC generated using IFN-α co-ordinately promote Type-1 anti-tumor immunity and decrease the potency of Treg responses in vitro (Gigante et al., 2008). These findings may have major implications for the development of therapeutic DC-based vaccines that focus on the resolution of uncontrolled DC activation.
Acknowledgements
This work was supported in part by grants: “Progetto Giovani Ricercatori” (2005) from University of Foggia, awarded to Margherita Gigante, and “Progetto Strategico della Regione Puglia (PS_012)”, 2006 to 2008, awarded to Elena Ranieri.
We are also grateful to Dr. Elisabetta Cavalcanti for critical reading of the manuscript.
References
- Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- de Vries IJ, Lesterhuis WJ, Scharenborg NM, Engelen LP, Ruiter DJ, Gerritsen MJ, Croockewit S, Britten CM, Torensma R, Adema GJ, Figdor CG, Punt CJ. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res. 2003;9:5091–5100. [PubMed] [Google Scholar]
- Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- Jonuleit H, Kuhn U, Muller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigante M, Mandic M, Wesa AK, Cavalcanti E, Dambrosio M, Mancini V, Battaglia M, Gesualdo L, Storkus WJ, Ranieri E. Interferon-alpha (IFN-alpha)-conditioned DC preferentially stimulate type-1 and limit Treg-type in vitro T-cell responses from RCC patients. J Immunother. 2008;31:254–262. doi: 10.1097/CJI.0b013e318167b023. [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Shinohara H, Miyamoto A, Okuzawa M, Mabuchi H, Nohara T, Gon G, Toyoda M, Tanigawa N. Prognostic value of tumor-infiltrating dendritic cells expressing CD83 in human breast carcinomas. Int J Cancer. 2003;104:92–97. doi: 10.1002/ijc.10915. [DOI] [PubMed] [Google Scholar]
- Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;5:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbeck PC, Kaveggia FF, Johansson SL, Grune MT, Taylor RJ. The relationships among tumor-infiltrating lymphocytes, histopathologic findings, and long-term clinical follow-up in renal cell carcinoma. Mod Pathol. 1992;5:420–425. [PubMed] [Google Scholar]
- Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, Delahunt B, Eble JN, Fleming S, Ljungberg B, Medeiros LJ, Moch H, Reuter VE, Ritz E, Roos G, Schmidt D, Srigley JR, Störkel S, van den Berg E, Zbar B. The Heidelberg classification of renal cell tumors. J Pathos. 1997;183:131–133. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kudo D, Rayman P, Horton C, Cathcart MK, Bukowski RM, Thornton M, Tannenbaum C, Finke JH. Gangliosides expressed by the renal cell carcinoma cell line SK-RC-45 are involved in tumor-induced apoptosis of T cells. Cancer Res. 2003;63:1676–1683. [PubMed] [Google Scholar]
- Ladányi A, Kiss J, Somlai B, Gilde K, Fejos Z, Mohos A, Gaudi I, Tímár J. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother. 2007;56:1459–1469. doi: 10.1007/s00262-007-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechmann M, Krooshoop DJEB, Dudziak D, Kremmer E, Kuhnt C, Figdor CG, Schuler G, Steinkasserer A. The extracellular domain of CD83 inhibits dendritic cell-mediated T cell stimulation and binds to a ligand on dendritic cells. J. Exp. Med. 2001;194:1813–1821. doi: 10.1084/jem.194.12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Torisu-Itakara H, Cochran AJ, Kadison A, Huynh Y, Morton DL, Essner R. Quantitative analysis of melanoma-induced cytokine-mediated immunosuppression in melanoma sentinel nodes. Clin Cancer Res. 2005;11(1):107–112. [PubMed] [Google Scholar]
- Lusini L, Tripodi SA, Rossi R, Giannerini F, Giustarini D, del Vecchio MT, Barbanti G, Cintorino M, Tosi P, Di Simplicio P. Altered glutathione anti-oxidant metabolism during tumor progression in human renal-cell carcinoma. Int J Cancer. 2001;91:55–59. doi: 10.1002/1097-0215(20010101)91:1<55::aid-ijc1006>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- Movassagh M, Spatz A, Davoust J, Lebecque S, Romero P, Pittet M, Rimoldi D, Liénard D, Gugerli O, Ferradini L, Robert C, Avril MF, Zitvogel L, Angevin E. Selective accumulation of mature DC-Lamp+ dendritic cells in tumor sites is associated with efficient T-cell-mediated antitumor response and control of metastatic dissemination in melanoma. Cancer Res. 2004;64:2192–2198. doi: 10.1158/0008-5472.can-03-2969. [DOI] [PubMed] [Google Scholar]
- O’Doherty U, Peng M, Gezelter S, Swiggard WJ, Betjes M, Bhardwaj N, Steinman RM. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- Ochsenbein AF, Riddell SR, Brown M, Corey L, Baerlocher GM, Lansdorp PM, Greenberg PD. CD27 expression promotes long-term survival of functional effector-memory CD8+ cytotoxic T lymphocytes in HIV-infected patients. J Exp Med. 2004;200:1407–1417. doi: 10.1084/jem.20040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patard JJ, Leray E, Rodriguez A, Rioux-Leclercq N, Guille F, Lobel B. Correlation between symptom graduation, tumor characteristics and survival in renal cell carcinoma. Eur Urol. 2003;44:226–232. doi: 10.1016/s0302-2838(03)00216-1. [DOI] [PubMed] [Google Scholar]
- Perrot I, Blanchard D, Freymond N, Isaac S, Guibert B, Pachéco Y, Lebecque S. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J Immunol. 2007;178:2763–2769. doi: 10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- Poindexter NJ, Sahin A, Hunt KK, Grimm EA. Analysis of dendritic cells in tumor-free and tumor-containing sentinel lymph nodes from patients with breast cancer. Breast Cancer Res. 2004;6:408–441. doi: 10.1186/bcr808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DJ, Jr, Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effectorT cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–250. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert TE, Scheuer C, Day R, Wagner W, Whiteside TL. The number of intratumoral dendritic cells and ζ-chain expression in T cells as prognostic and survival biomarkers in patients with oral carcinoma. Cancer. 2001;91:2136–2147. [PubMed] [Google Scholar]
- Reid SD, Penna G, Adorini L. The control of T cell responses by dendritic cell subsets. Curr. Opin. Immunol. 2000;12:114–121. doi: 10.1016/s0952-7915(99)00059-x. [DOI] [PubMed] [Google Scholar]
- Saint-Vis B, Vincent J, Vandenabeele S, Vanbervliet B, Pin JJ, Ait-Yahia S, Patel S, Mattei MG, Banchereau J, Zurawski S, Davoust J, Caux C, Lebecque S. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–336. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–1361. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Stoitzner P, Green LK, Jung JY, Price KM, Atarea H, Kivell B, Ronchese F. Inefficient presentation of tumor-derived antigen by tumor-infiltrating dendritic cells. Cancer Immunol Immunother. 2008 doi: 10.1007/s00262-008-0487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarkiewicz J, Rybojad P, Jablonka A, Rolinski J. CD1c+ and CD303+ dendritic cells in peripheral blood, lymph nodes and tumor tissue of patients with non-small cell lung cancer. Oncol Rep. 2008;19:237–243. [PubMed] [Google Scholar]
- Tatsumi T, Kierstead LS, Ranieri E, Gesualdo L, Schena FP, Finke JH, Bukowski RM, Mueller-Berghaus J, Kirkwood JM, Kwok WW, Storkus WJ. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE- 6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196:619–628. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi T, Kierstead LS, Ranieri E, Gesualdo L, Schena FP, Finke JH, Bukowski RM, Brusic V, Sidney J, Sette A, Logan TF, Kasamon YL, Slingluff CL, Jr, Kirkwood JM, Storkus WJ. MAGE-6 encodes HLA-DRbeta1*0401-presented epitopes recognized by CD4+ T cells from patients with melanoma or renal cell carcinoma. Clin Cancer Res. 2003;9:947–954. [PubMed] [Google Scholar]
- Van den Hove LE, Van Gool SW, Van Poppel H, Baert L, Coorevits L, Van Damme B, Ceuppens JL. Phenotype, cytokine production and cytolytic capacity of fresh (uncultured) tumour-infiltrating T lymphocytes in human renal cell carcinoma. Clin Exp Immunol. 1997;109:501–509. doi: 10.1046/j.1365-2249.1997.4771375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]