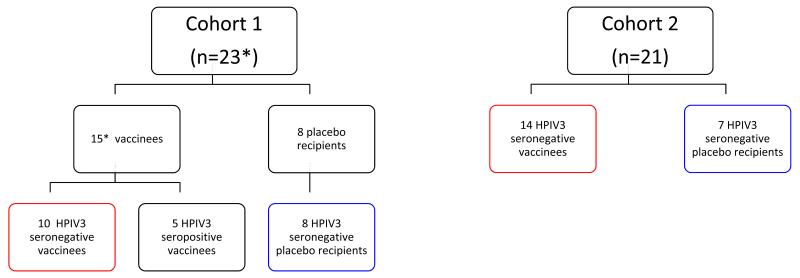
Figure 1.
Enrollment into the phase 1 clinical trial of the rcp45 vaccine. *, evaluable subjects. As noted in the text, a single vaccinated HPIV3 seronegative child in cohort 1 was withdrawn by his parents on study day 10, so that data from 23 of 24 children in cohort 1 were included. For purposes of reporting, clinical data from all HPIV3 seronegative vaccinees (n=24, groups outlined in red) and all HPIV3 seronegative placebo recipients (n=15, groups outlined in blue) were combined in Table 1.