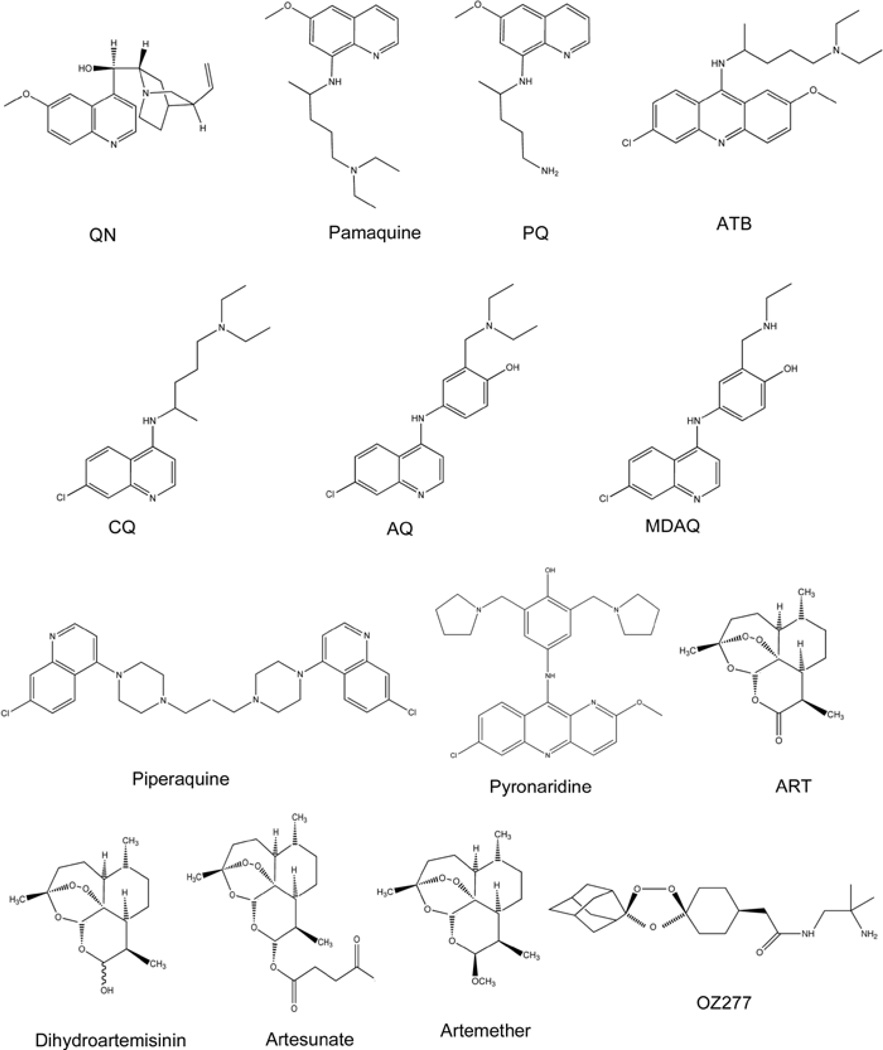
Figure 1. Chemical structures of antimalarial drugs inspired by the active compounds of cinchona bark and qinghao.
Antimalarials inspired by the active compounds of the cinchona bark are characterized by the presence of a quinoline heteroaromatic nucleus. Represented quinoline antimalarials include: QN, a quinoline methanol; the 8-aminoquinolines pamaquine and PQ; the acridine-based compounds ATB and pyronaridine; and the 4-aminoquinolines CQ, AQ, its active metabolite MDAQ, and the bisquinoline piperaquine. It is suggested that the target of most quinoline antimalarials is haematin (aquaferriprotoporphyrin IX), an autoxidized haem released during haemoglobin degradation and found as crystallized dimers in the acidic vacuoles of infected red blood cells of Plasmodium parasites. Most quinoline drugs complex with haematin, which is thought to kill the parasite by an oxidative or osmotic mechanism. Antimalarials inspired by the active compounds of qinghao include the sesquiterpene lactone ART, and its derivatives dihydroartemisinin, artesunate and artemether. The endoperoxide bridge is crucial for its antiparasitic activity and is proposed to cause oxidative stress by the formation of ROS. A recent fully synthetic endoperoxide antimalarial inspired by ART is arterolane (OZ277), which presents a spiroadamantane trixoloane pharmacophore and neutral or basic functional groups designed to improve oral bioavailability and increase its half-life.