Table 1.
In vitro GSK-3β inhibition by the piperidyl maleimides.[a]
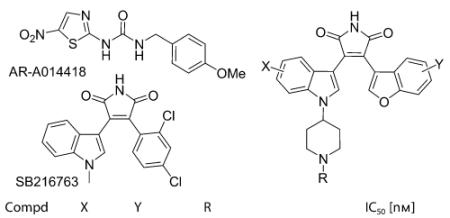
| Compd | X | Y | R | IC50 [nm] |
|---|---|---|---|---|
| 3a | H | H | H | 67±6 |
| 3b | H | 6-OMe | H | 629±32 |
| 3c | H | 7-OMe | H | 134±5 |
| 3d | 5-F | H | H | 30±4 |
| 3e | 5-Cl | H | H | 272±27 |
| 3 f | 5-Br | H | H | 684±17 |
| 3g | 5-F | 7-OMe | H | 28±4 |
| 3h | H | H | CH3 | 155±2 |
| 3i | H | H | CH2(2-pyridyl) | 110±4 |
| 3j | H | H | CH2(4-pyridyl) | 65±4 |
| 3k | H | H | CO(1-morpholinyl) | 10±2 |
| 3l | H | H | CO(2-pyridyl) | 6±1 |
| 3m | H | H | CO(3-pyridyl) | 8±2 |
| 3n | H | H | CO(4-pyridyl) | 4±1 |
| 3o | H | H | CO(2-pyrazyl) | 4±1 |
| 3p | H | H | COCH2NH2 | 55±2 |
| 3q | H | H | CO(4-methylfurazanyl) | 25±2 |
| 1 | – | – | – | 87±24[b] |
| Staurosporine | 12±8 | |||
| SB216763 | 23±1 | |||
| AR-A014418 | 229±11 | |||
The synthesized maleimides were evaluated for their ability to inhibit phosphorylation of primed substrate (YRRAAVPPSPSLSRHSSPHQ(pS)E DEEE; 20 μm) by human GSK-3β in the presence of 10 μm ATP concentration. These compounds were tested at Reaction Biology Inc. (http://www.reactionbiology.com).
An IC50 value of 7±3 nm was obtained when compound 1 was tested using the conditions reported in Ref. [13a].
