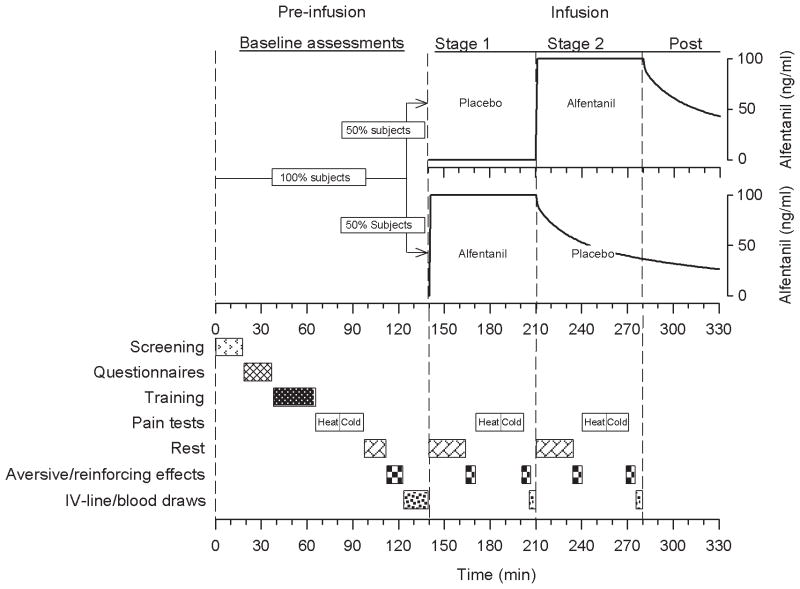
Figure 1.
Two hundred and twenty eight monozygotic and dizygotic twins successfully underwent a computer-controlled infusion with the μ-opioid agonist alfentanil in a single occasion, randomized, double-blinded and placebo-controlled study paradigm. Baseline assessments included respiratory parameters (transcutaneous carbon dioxide and respiratory rate), cognitive speed, pain tests (reported elsewhere), covariates potentially affecting measured opioid effects (demographics, psychometric tests, sleep quality), vital signs, and blood draws. Fifty percent of twin pairs were allocated to receive alfentanil first and saline placebo second, while the other 50% of twin pairs received alfentanil and saline placebo in reversed order. The alfentanil target concentrations for both treatment sequences are depicted in the graph. A concentration of 100 ng/ml produces significant analgesic and aversive opioid effects in patients suffering from postoperative pain. Respiratory parameters, cognitive speed, subjective aversive effects (nausea, dizziness, sedation, pruritus), reinforcing effects (drug liking and disliking), analgesic effects (reported elsewhere), and vital signs were assessed in identical fashion during both stages of the infusion protocol. Blood draws for assaying alfentanil plasma concentrations were also obtained. This figure has been reproduced with permission of the International Association for the Study of Pain® (IASP®). The figure may not be reproduced for any other purpose without permission.