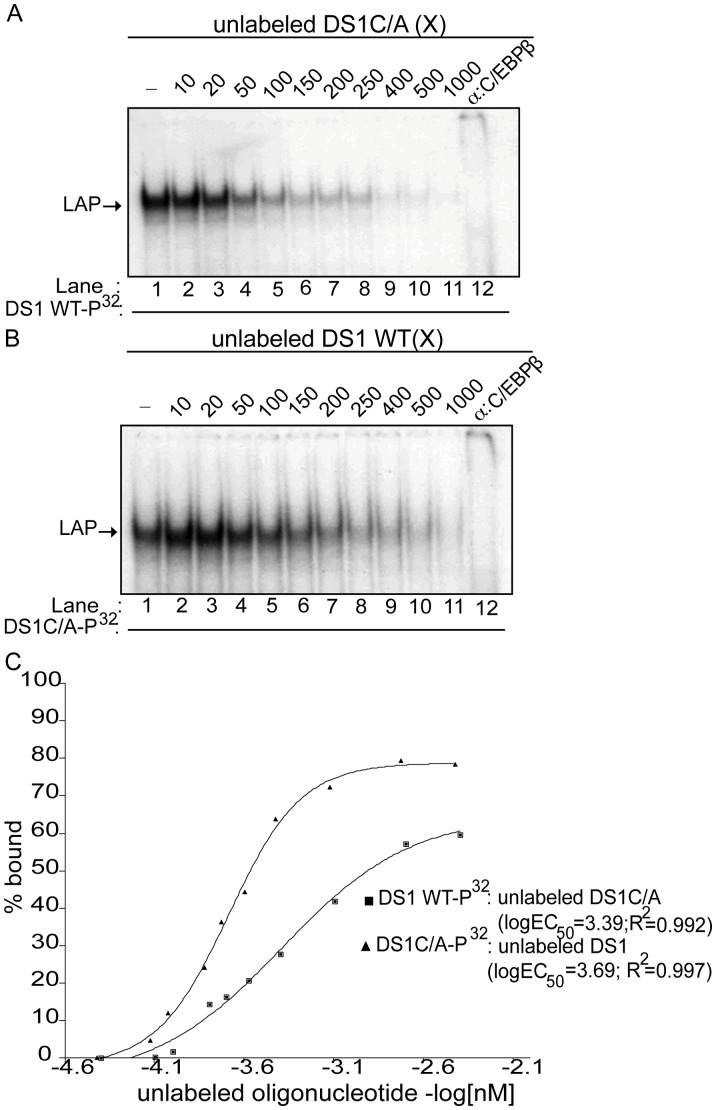
Figure 5. The DS1C/A site has a two-fold increased affinity for LAP compared to the DS1 wild-type C/EBP site.
(A) Competition EMSA using nuclear extract obtained from HEK-293T cells transfected with the pCMV-LAP construct and incubated with labeled DS1 wild-type (WT) oligonucleotide alone (lane 1); 10 to 1000-fold molar excess (X) unlabeled DS1C/A oligonucleotide as indicated (lanes 2–11); or incubated with labeled DS1WT oligonucleotide and anti-C/EBPβ antibody (lane 12). (B) Competition EMSA using nuclear extract obtained from HEK-293T cells transfected with the pCMV-LAP construct and incubated with labeled DS1C/A oligonucleotide alone (lane 1); 10 to 1000-fold molar excess (X) unlabeled DS1WT oligonucleotide (lanes 2–11); or labeled DS1C/A oligonucleotide and anti-C/EBPβ antibody (lane 12). (C) Concentration of unlabeled oligonucleotide (-log[nM]) versus the percentage (%) of LAP bound to the labeled oligonucleotide, as determined by densitometric analysis of band intensities from the competitive EMSAs (A and B), was plotted. A non-linear regression curve was used to determine the KD of LAP for the DS1 WT and DS1C/A sites from the logEC50 (half maximal protein binding) values as described in the Materials and Methods. Shown are representative EMSAs (n = 2).