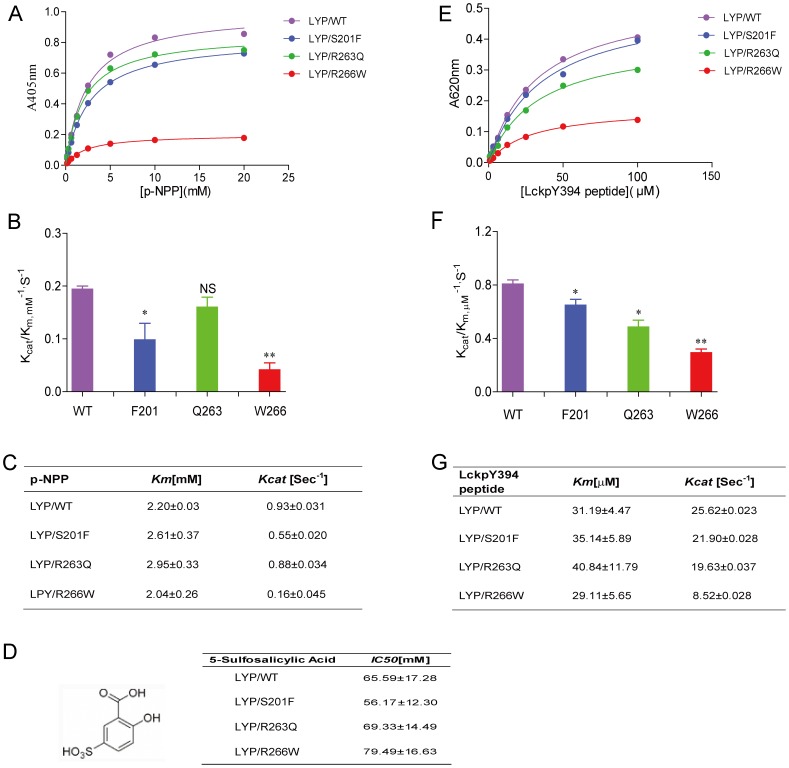
Figure 2. Kinetic analysis of the phosphatase activity of Lyp catalytic domain wild type and S201F, R263Q and R266W mutants toward pNPP and phosphor-peptide.
(A) pNPP hydrolysis with Lyp catalytic domain domain and 3 mutants. Data were fitted to the Michaelis-Menten equation. (B–C) Kinetics parameters for the wild-type and the mutants of Lyp toward pNPP. All experiments were repeated four times. * represents P<0.05; ** represents P<0.01. (D) IC50 values of 5- sulfosalicylic acid toward Lyp wild type and variants. (E) Lyp catalyzed phosphate release of a 9 amino acid phosphor peptide derived from Lck 394 phosphorylation site (EDNEpYTARE). Data were fitted to the Michaelis-Menten equation. (F–G) Kinetics parameters for the wild-type and the mutants of Lyp toward EDNEpYTARE. Data showed the mean of three independent experiments. *P<0.05; ** P<0.01.