Abstract
Increasing partial pressure of atmospheric CO2 is causing ocean pH to fall—a process known as ‘ocean acidification’. Scenario modeling suggests that ocean acidification in the Baltic Sea may cause a ≤3 times increase in acidity (reduction of 0.2–0.4 pH units) by the year 2100. The responses of most Baltic Sea organisms to ocean acidification are poorly understood. Available data suggest that most species and ecologically important groups in the Baltic Sea food web (phytoplankton, zooplankton, macrozoobenthos, cod and sprat) will be robust to the expected changes in pH. These conclusions come from (mostly) single-species and single-factor studies. Determining the emergent effects of ocean acidification on the ecosystem from such studies is problematic, yet very few studies have used multiple stressors and/or multiple trophic levels. There is an urgent need for more data from Baltic Sea populations, particularly from environmentally diverse regions and from controlled mesocosm experiments. In the absence of such information it is difficult to envision the likely effects of future ocean acidification on Baltic Sea species and ecosystems.
Keywords: pH, CO2, Cod, Herring, Mytilus, Spring bloom, Phytoplankton, Skagerrak, Kattegat
Introduction
The open oceans act as a major buffer for atmospheric gases. Of the atmospheric CO2 that has been released by mankind since the onset of industrialisation ~30 % has been absorbed by the oceans. This has caused the average pH of the open oceans to fall by 0.1 units (IPCC 2007)—a seemingly small change, but one that is equivalent to a ca. 25 % increase in acidity. The underlying chemical processes are well understood (see Box), and consequently unlike climate scenarios, future changes in ocean pH can be predicted reliably from scenarios of future atmospheric CO2 concentrations. Those scenarios project possible reductions in open ocean pH of as much as 0.35 units (equivalent to a 3 times increase in acidity) within the coming 100 years (Cao et al. 2007; IPCC 2007).
Closer to shore the effects of atmospheric CO2 on coastal seawater pH are complicated by local effects of run-off, eutrophication, upwelling, atmospheric deposition and remineralisation, all of which also influence local biogeochemistry (Doney et al. 2007; Omstedt et al. 2009, 2010). Consequently, coastal pH is more variable and difficult to predict than that of the open ocean (Andersson et al. 2005). Data from coastal regions in the southern Baltic Sea reflect this: pH of the Kiel fjord varies by ca. 0.7 pH units seasonally (Thomsen et al. 2010), and diurnal fluctuations of ±0.15 pH units are common in shallow bays of the Skagerrak (personal observation). Similar observations have been made in a variety of open ocean and coastal locations (Wootton et al. 2008; Hofmann et al. 2011). These diurnal changes—and a substantial fraction of the seasonal changes—in pH are driven by direct effects of photosynthesis and respiration. Until relatively recently such variability in seawater pH had been largely overlooked in ocean acidification literature, however, it is now clear that temporal variation in pH is a pervasive natural feature of marine systems. The effects of superimposing near-future ocean acidification on this variation are as yet unknown, but it seems clear that many marine organisms may already be adapted to more pH variability than was previously thought.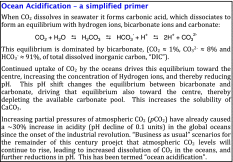
The capacity of seawater to buffer the addition of weak acids (such as H2CO3) is directly related to the total alkalinity, which is closely related to the salinity. Seawater in the Skagerrak–Kattegat–Baltic Sea system has reduced alkalinity and therefore reduced buffering capacity, with the result that pH is more variable (and generally lower) than in the open ocean. For example, annual pH range for surface waters in the central Kattegat (SMHI Station Anholt E) for the period 1992–2007 was 8.06–8.42 pH units, for the less saline central Baltic Sea (SMHI Station BY15) was 8.02–8.70 pH units, and for the very brackish northern Bothnian Bay (SMHI Station F9) was 7.40–8.37 pH units (SMHI 2011). Future increases in atmospheric CO2 likely cause substantial shifts in pH in these waters, especially at lower salinities and alkalinities. Climate scenario projections for the end of the century suggest that surface water pH in the central Baltic Sea will fall by ca. 0.25 pH units (Kuznetsov et al. unpublished results) or up to 0.45 pH units (Omstedt et al. unpublished results). In deep water, modeling suggests increased spatial extent of anoxia, which provides increased buffering against ocean acidification. Nonetheless, modeling shows that pH in deep waters of the central Baltic Sea, which is already very low (typically ≤7.3 units), is likely to fall by at least an additional 0.1 pH units (Omstedt et al. unpublished results). This considerable variation in range overlaps the ca. 0.35 pH unit change anticipated in the open oceans under the same scenario (Cao et al. 2007; IPCC 2007), and represents a substantial shift in pH.
Research into the biological effects of ocean acidification has expanded rapidly in the last 5 years. Several reviews now show that the effects of ocean acidification vary markedly between different classes of organism, between closely related species, and between life stages within the same species (Doney et al. 2009, 2012; Byrne 2011). More detailed meta-analyses of published results have drawn divergent conclusions, although some prominent examples have been based on flawed methodologies. A recent comprehensive meta-analysis (Kroeker et al. 2010) concluded that overall effects on marine animals are negative, while effects on marine primary producers are generally positive. The overriding result from this analysis, however, was that substantial variation exists among the responses of different taxa and processes to acidification (e.g. corals respond differently to fishes, and effects on reproduction differ from those on survival; Kroeker et al. 2010). None of these reviews have evaluated intraspecific variation in responses to ocean acidification, and yet it is precisely this variation that provides the raw materials for the acclimation (i.e. plasticity) and adaptation (i.e. change in gene frequencies) that will be required if marine species are to survive near-future climate change.
This article reviews the available data on effects of ocean acidification on Baltic Sea marine species, focusing in particular on those taxa that modeling suggests have the greatest influence on the Baltic Sea foodweb (Niiranen et al. 2012; Fig. 1). In many cases, the effects of ocean acidification on these species have not been studied in Baltic Sea populations, and therefore summaries are provided from studies conducted elsewhere (note that the near complete absence of data precludes consideration of several ecologically important taxa such as heterotrophic bacteria, viruses and nanoflagellates). Problems involved in up-scaling from population- and species-level studies to draw ecosystem-level inferences are highlighted, and recommendations are made for future research and analyses. Where possible, differences in responses of species in the different parts of the Baltic Sea are noted (although again, available data are very few). Throughout, the general term ‘Baltic Sea’ will be used to refer to the whole Skagerrak–Kattegat–Baltic Sea system, whereas specific areas within this system will be referred to by name (e.g. “Central Baltic Sea”).
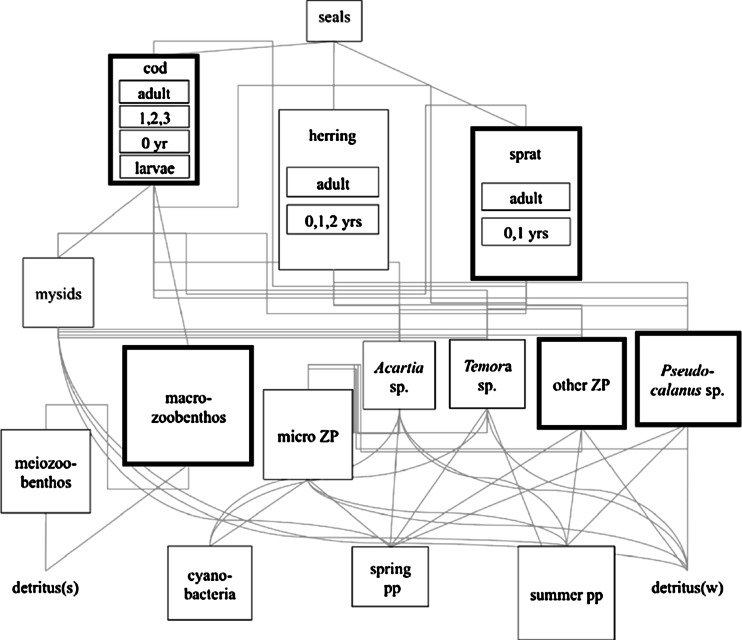
Fig. 1.
Flow chart of the BalProWeb model (Tomczak et al. 2012). Bold boxes indicate groups with greatest influence on model results (Niiranen et al. 2012). The effects of ocean acidification on these groups is the focus of this article (ZP zooplankton, PP phytoplankton, detritus(s) sediment detritus and detritus(w) water-column detritus). From Tomczak et al., modified
Effects of Ocean Acidification on Baltic Sea Species
Few studies have investigated the effects of ocean acidification on Baltic Sea species and populations, although many studies are now in progress and data are becoming available. Interpopulation variability in response to ocean acidification is to be expected as a consequence of adaptation to local conditions, and several authors have shown that this can be substantial in some species (Findlay et al. 2010a, b; Walther et al. 2010, 2011). It must be remembered, however, that the prevalence of such examples may not represent the prevalence of trait variability as there are likely to be biases against reporting of non-significant differences between populations, which have also been found (Havenhand and Kurihara, unpublished results). Nonetheless, given the special nature of the Baltic Sea ecosystem, caution should be used when using results obtained from populations outside the Baltic Sea to draw inferences about the likely effects of ocean acidification on species inside the Baltic Sea system.
Primary Producers
The positive effects of ocean acidification on plants and algae reported from meta-analyses (Kroeker et al. 2010) are reflected in data available for Baltic Sea species. In the eelgrass Zostera marina from the Kattegat, ocean acidification (≈72 μatm CO2) increased shoot biomass in mesocosms (Eklöf et al. 2012). In studies of populations of Z. marina from the Pacific, photosynthesis, below-ground productivity and flowering density have all been shown to increase under elevated CO2 (Zimmerman et al. 1997; Palacios and Zimmerman 2007). At the pCO2 levels expected in the near-future (≤1000 μatm; Cao et al. 2007; IPCC 2007) the magnitude of these responses is relatively small (Kroeker et al. 2010; Eklöf et al. 2012). Nonetheless, eelgrass growth will likely benefit from local ocean acidification (see “Ecosystem effects” below).
Benthic macroalgae are also important primary producers and ecosystem engineers in Baltic Sea ecosystems, however, very few reports in the literature detail the effects of ocean acidification on macroalgae. Most macroalgae have CO2 concentrating mechanisms (CCMs), which reduce their dependency on ambient CO2 (or HCO3−) concentrations. Therefore, these taxa have been assumed to be largely insensitive to acidification (Giordano et al. 2005). There are no published results for the effects of ocean acidification on Baltic Sea macroalgae, but recent work elsewhere confirms that species with CCMs are likely to be unaffected by, or may benefit marginally from, ocean acidification (Hepburn et al. 2011). These authors also note that the responses to ocean acidification of macroalgae, such as the Fucus spp. common in the Baltic Sea, will be highly dependent on light intensities. Consequently, the balance between direct effects of pCO2 on macroalgal growth and the indirect effects on macroalgal photosynthesis via planktonic microalgal growth, water clarity, and hence light levels, will determine the overall outcome of ocean acidification for benthic macroalgae in the Baltic Sea.
For planktonic microalgae, early work on ecosystem-scale pCO2 effects in the Baltic Sea suggested that seasonal under-saturation of calcite1 may explain the absence of calcifying coccolithophores from Baltic Sea waters (Tyrrell et al. 2008). Seasonally high pCO2 levels such as those noted by Tyrrell et al. (2008) are typically the result of remineralisation of organic material rather than a direct result of ocean acidification. But ocean acidification is likely to augment these effects (see above), and therefore the capacity for seasonal limitation of species distributions due to high pCO2 (and accordant reduced pH) is likely to increase.
Unlike the open ocean, where substantial blooms of coccolithophores can be an important component of microalgal production, Baltic Sea phytoplankton communities are dominated by spring blooms of diatoms, and summer blooms of cyanobacteria (“blue-green algae”). Here again, relatively little work has been done on Baltic Sea species or populations. The prevalence of CCMs in phytoplankton has been suggested as a reason to expect reduced sensitivity to ocean acidification (Giordano et al. 2005; Hopkinson et al. 2011). For spring bloom species, recent results for the diatom Skeletonema marinoi from the Skagerrak showed that the effects of ocean acidification on growth rate differed between strains and populations but overall, growth of S. marinoi was not affected by increased pCO2 (750 μatm; Kremp et al. unpublished results). Studies from other regions have shown that diatom growth can benefit slightly from higher pCO2 levels, and when placed in competition with calcifying phytoplankton acidification led to increased competitive ability (Riebesell et al. 2007). Clearly, available evidence is scant, but on balance it seems likely that the effects of ocean acidification on the Baltic Sea spring phytoplankton bloom will be small, and perhaps positive.
The effects of ocean acidification on cyanobacteria—the major component of the summer phytoplankton bloom in the Baltic Sea—are slightly better understood. In Nodularia spumigena, a common toxic species in the Baltic Sea, Czerny et al. (2009) found reduced cell-division rates and increased heterocyst production as pCO2 increased over a wide range (160–730 μatm). More recent work found no effect of elevated pCO2 (970 μatm) on biovolume or specific growth rate for N. spumigena from the central Baltic Sea (Karlberg and Wulff 2012). Similar non-significant results were found for Aphanizomenon sp., another dominant Baltic Sea cyanobacteria (Karlberg and Wulff 2012). Czerny et al. (2009) suggest that the patterns they observed may be a result of adaptations to the substantial diurnal variation in pH (greater than or equal to ±1 pH unit) that can occur in blooms of these algae in the Baltic Sea (Ploug 2008). Again, there is relatively little available evidence, but it seems probable that ocean acidification will have no, or a minor negative, effect on summer phytoplankton blooms in the Baltic Sea.
Perhaps most interestingly, the effects of ocean acidification on phytoplankton may be mediated through processes other than growth. Recent study has shown that the dinoflagellate Alexandrium ostenfeldii, a common (although typically not bloom forming) species in the central Baltic Sea, produces higher levels of saxitoxin under elevated pCO2 (Kremp et al. unpublished results). Similar results for other dinoflagellates have been found in other parts of the world (Fu et al. 2010). No study has yet been done on the effects of increased pCO2 on the common toxic bloom-forming dinoflagellates in the Baltic Sea, although these findings herald the possibility that near-future climate change may increase the toxicity of at least some of the algal blooms in the Baltic Sea.
Zooplankton
Zooplankton form a key component of the Baltic Sea food web, however, once again there are no published data from Baltic Sea species or populations with which an assessment of the likely impacts of ocean acidification can be made. Reports from the North Sea suggest that increasing pCO2 will benefit gelatinous zooplankton (Attrill et al. 2007), but these conclusions are based on environmental correlation rather than experimental observations, and therefore cannot illustrate causality (Haddock 2008). Data for copepods from other parts of the world show that high pCO2 (2000–2300 μatm) did not influence survival, size, development, or egg production of Acartia steueri (Kurihara et al. 2004) or of Acartia tsuensis (Kurihara and Ishimatsu 2008), but that extreme values (8000 μatm) did influence development (though not growth and egg production) of Calanus finmarchicus (Mayor et al. 2007). Modeling suggests that the copepod Pseudocalanus sp. and ‘other mesozooplankton’ (excluding Pseudocalanus sp., Acartia spp. and Temora sp.) are particularly important for the Baltic Sea food web (Fig. 1). Given that the extreme pCO2 levels used in experiments to date elicited little or no response, and that the relatively rapid generation times of copepods confers a high potential for adaptability, it seems reasonable to expect that copepods will be resilient to near-future ocean acidification (≤1000 μatm CO2). Nonetheless, as noted earlier, the absence of data from Baltic Sea populations makes any conclusions about the direct effects of ocean acidification on zooplankton tentative.
Macrozoobenthos
Several studies have investigated the effects of ocean acidification on macrozoobenthos from the Baltic Sea. Most of these come from the Kiel fjord, where summer upwelling can drive the pH down to 7.5 (Thomsen et al. 2010), well below the levels typically predicted for the open oceans by end of the century (≤1000 μatm CO2; Cao et al. 2007; IPCC 2007). Here, the mussel Mytilus edulis, a dominant macrobenthic species in the Baltic Sea, was found to maintain shell and somatic growth rates at ca. 1400 μatm CO2 (≈pH 7.6). M. edulis was also observed to recruit actively at 1000 μatm CO2 (≈pH 7.75; Thomsen et al. 2010). Thomsen et al. (2010) suggest that this unusual ability to maintain physiological function despite substantial levels of acidification was due in part to an abundance of food, which provided the energy needed to offset the physiological costs of maintenance and growth at such low ambient pH (Melzner et al. 2011). More sensitive early life-history stages of M. edulis from the Skagerrak have been shown to respond differently to ocean acidification: fertilization success increased at reduced pH (induced by high pCO2) whereas subsequent larval shell growth was negatively affected, albeit only slightly (Renborg and Havenhand, unpublished results). Similar small negative effects on larval shell growth have also been reported in populations of M. edulis from the North Sea (Gazeau et al. 2010; Bechmann et al. 2011), and in related Mytilus species around the world (Kurihara et al. 2009; Gaylord et al. 2011; Sunday et al. 2011). Early reports of the effects of ocean acidification on shell growth in adult M. edulis showed negative impacts (Gazeau et al. 2007), a result that contrasts with those of Melzner and co-workers in the Kiel fjord (Thomsen et al. 2010; Melzner et al. 2011), although the latter might be expected to be a result of local adaptation to seasonally low pH—especially at such extreme levels (Melzner et al. 2009b; Thomsen et al. 2010).
Together with M. edulis, the clam Macoma balthica dominates the seafloor of much of the central and northern Baltic Sea. These two species comprise the overwhelming majority of the ‘macrozoobenthos’ category identified as having a marked effect on output from ecosystem models of the central Baltic Sea (Niiranen et al. 2012). Unfortunately, there are no available published data on the effects of ocean acidification on Macoma species, and therefore it is not possible to draw meaningful conclusions on the likely sensitivity of this key component of the Baltic Sea food web. It is clear, however, that tolerance of M. edulis to ocean acidification increases with available food ration (Melzner et al. 2011). Increased ocean acidification represents an additional stress on marine organisms that can cause corresponding increases in maintenance and physiological costs (Pörtner et al. 2004; Pörtner 2008). This ‘acidification stress’ will operate in concert with other stressors that limit the distribution and function of species—particularly salinity, which is a major determinant of mussel size and distribution in the Baltic Sea (Tedengren and Kautsky 1987; Westerbom et al. 2008). Food-dependent tolerance to ocean acidification has not only been observed in the mussel (Melzner et al. 2011), but also in barnacle, Balanus improvisus, from the Baltic Sea (Pansch, unpublished results), as well as in other species elsewhere. Consequently, the availability of adequate energy reserves in the form of food may determine tolerance to ocean acidification in many species of macrozoobenthos. This emphasises the need for multifactor experiments on multiple trophic levels (see “Ecosystem effects” below).
In summary, available evidence suggests changes in macrozoobenthos as a result of future ocean acidification will be small, however, there is considerable uncertainty in this assessment and many more data from additional populations throughout the Baltic Sea are required.
Fish
Cod, herring and sprat dominate fisheries in the Baltic Sea (Sparholt 1991; Heikinheimo 2011) and are key components of Baltic Sea food web models (Harvey et al. 2003). These food web models are highly sensitive to changes in cod and sprat abundances (Niiranen et al. 2012), suggesting that the effects of ocean acidification on these species may have more far-reaching consequences than for other components of the Baltic Sea food web. The rapidly growing body of data reporting the effects of ocean acidification on cod and herring species shows that these species appear to be relatively robust to considerable pCO2-mediated changes in pH. For example, sperm motility in Baltic Sea cod has been shown to be insensitive to moderate levels of acidification (≤1360 μatm CO2 ≈ pH 7.55; Frommel et al. 2010), leading these authors to conclude that fertilization success in cod would also be unaffected by near-future acidification. Similarly, fertilization, embryogenesis, hatching success and larval growth of Baltic Sea herring were unaffected by pCO2 up to 4600 μatm (Franke and Clemmesen 2011). At higher pCO2, RNA/DNA ratios were reduced suggesting that growth may be impaired, however, there were no statistically significant effects on any of the variables measured at pCO2s representative of IPCC scenarios for the years 2100 and 2200 (Franke and Clemmesen 2011). As for the M. edulis example discussed earlier, these herring data come from the Kiel fjord, and Franke and Clemmesen (2011) note that their results may reflect adaptation of this population to increasing pCO2 in the surface waters during larval development.
Data from Norwegian cod populations show that tolerance to ocean acidification declines gradually early larval stages, leading to some organ damage in later larvae and juveniles (Frommel et al. 2012). Melzner et al. (2009a), also using Norwegian cod, exposed juveniles reared under normal pCO2 conditions to elevated (3000 μatm) pCO2 for several months, and found no effect on metabolic rates and critical swimming speeds (Melzner et al. 2009a). The consequences of the changes observed by Frommel et al. (2012) on swimming performance of later juveniles are not known. Equivalent data for herring and sprat are currently lacking; however, on balance, it seems likely that the effects of ocean acidification on cod and herring will be small, although negative impacts may be experienced in later larval stages. Once again, experimental data from Baltic Sea populations are needed in order to reduce the uncertainties around this estimate.
In a much broader ecosystem context, dynamic climate envelope modeling of the effects of climate change on global fisheries suggests that an overall lack of (or low) sensitivity to ocean acidification (as observed here for most species and life stages) will result in no net change to maximum catch potential in Baltic Sea fisheries by 2050 (Cheung et al. 2011). However, Cheung et al. (2011) caution that if commercially important Baltic Sea fish species are in fact sensitive to ocean acidification, then maximum catch potential may fall by up to 30 %.
Ecosystem Effects
Responses of ecosystems to ocean acidification will be influenced by multiple abiotic factors (notably temperature and salinity) as well as by interactions between species. The effects of ocean acidification on competition, predation and mutualisms are only beginning to be understood, and examples are few. At the time of going to press there are no published studies that have investigated the effects of multiple climate change variables on multiple trophic levels in the Baltic Sea region, although Eklöf et al. (2012) investigated the effects of ocean acidification and warming on seagrass mesocosms in the Skagerrak. They found substantial effects of warming, and small positive effects of acidification on seagrasses and macroalgae, and neutral effects on grazers in the system. Importantly, these impacts were context dependent: acidification amplified the effects of warming, but only in the absence of grazers (Eklöf et al. 2012). This result could not have been predicted from single-species studies, highlighting the need for multifactor multispecies approaches. Equivalent patterns can be found in literature on the effects of ocean warming (e.g. Kordas et al. 2011), and therefore it seems clear that changing abiotic factors will alter competitive, and trophic, interactions of key functional groups (Hepburn et al. 2011). Understanding these changes will be fundamental to understanding how ecosystems will respond in a high CO2 ocean.
Conclusions
The responses of marine organisms to ocean acidification vary markedly between populations, between species and between life-history stages. Consequently, drawing conclusions as to the likely effects of ocean acidification is problematic at best. For Baltic Sea ecosystems conclusions are severely constrained by the lack of experimental and observational data for many functional groups. Available data from experiments that have used Baltic Sea populations show that many key taxa in the food web of the Baltic Sea are generally tolerant of the pCO2-mediated pH excursions expected in the coming century, or respond only slightly. Exceptions to this pattern are larval stages of mussels and cod, which may experience biologically significant negative impacts. There is, however, a paucity of data and future studies may find more significant effects.
In attempting to place these findings in context it must be remembered that ocean acidification will operate in concert with other environmental variables to change competitive, predator and mutualistic interactions among species. This will in turn reshape the fitness and selection landscapes in ways that the single-factor, single-species experiments that have dominated the ocean acidification literature cannot inform. How Baltic Sea species respond to these changed landscapes will depend on their capacity for acclimation and adaptation (plasticity and genetic change, respectively) in response to all the stresses placed upon them, not solely to ocean acidification. To use a colloquial analogy, when two friends out walking in the forest were suddenly confronted with a charging bear and one of them started to run, the other shouted “Why run? The bear can outrun us both!” His friend responded “I don’t need to run faster than the bear, I only need to run faster than you!” It is not the absolute response to ocean acidification, but the balance between fitness costs and fitness benefits of ocean acidification that will determine winners and losers in a future high CO2 Baltic Sea. At present, there are too few data to be able to state with any degree of certainty which taxa will fall into which category.
Acknowledgments
Andrea Frommel, Anna Godhe, Ivan Kuztnetsov, Anders Omstedt and Angela Wulff, all generously shared unpublished data, and Sam Dupont pointed me in the direction of some useful ocean acidification literature that I’d missed. Thanks to all. I’m particularly grateful to Ann-Turi Skjevik, who provided valuable insights on the algal bloom dynamics of the Baltic Sea. This study was supported by the project ECOSUPPORT (Advanced modeling tool for scenarios of the Baltic Sea ECOsystem to SUPPORT decision making) under the EU 7th Framework Programme (FP/2007-2013) BONUS programme, and was partly undertaken within the Linnaeus Centre for Marine Evolutionary Biology (http://www.cemeb.science.gu.se/), supported by a Linnaeus-grant from the Swedish Research Councils VR and Formas.
Jonathan N. Havenhand
is a Professor and Researcher in the Department of Biological & Environmental Sciences at the University of Gothenburg, Sweden. He is based at the Sven Lovén Centre for Marine Sciences, Tjärnö, where he works on fertilization success and larval ecology of marine invertebrates.
Footnotes
Calcite is a mineral form of CaCO3, the saturation state of which decreases with increasing ocean acidification.
References
- Andersson AJ, Mackenzie FT, Lerman A. Coastal ocean and carbonate systems in the high CO(2) world of the anthropocene. American Journal of Science. 2005;305:875–918. doi: 10.2475/ajs.305.9.875. [DOI] [Google Scholar]
- Attrill MJ, Wright J, Edwards M. Climate-related increases in jellyfish frequency suggest a more gelatinous future for the North Sea. Limnology and Oceanography. 2007;52:480–485. doi: 10.4319/lo.2007.52.1.0480. [DOI] [Google Scholar]
- Bechmann RK, Taban IC, Westerlund S, Godal BF, Arnberg M, Vingen S, Ingvarsdottir A, Baussant T. Effects of ocean acidification on early life stages of shrimp (Pandalus borealis) and mussel (Mytilus edulis) Journal of Toxicology and Environmental Health-Part a. 2011;74:424–438. doi: 10.1080/15287394.2011.550460. [DOI] [PubMed] [Google Scholar]
- Byrne M. Impact of ocean warming and ocean acidification on marine invertebrate life history stages: Vulnerabilities and potential for persistence in a changing ocean. Oceanography and Marine Biology: An Annual Review. 2011;49:1–42. [Google Scholar]
- Cao, L., K. Caldeira, and A.K. Jain. 2007. Effects of carbon dioxide and climate change on ocean acidification and carbonate mineral saturation. Geophysical Research Letters 34. doi:10.1029/2006GL028605.
- Cheung WWL, Dunne J, Sarmiento JL, Pauly D. Integrating ecophysiology and plankton dynamics into projected maximum fisheries catch potential under climate change in the Northeast Atlantic. ICES Journal of Marine Science. 2011;68:1008–1018. doi: 10.1093/icesjms/fsr012. [DOI] [Google Scholar]
- Czerny J, Ramos JBE, Riebesell U. Influence of elevated CO(2) concentrations on cell division and nitrogen fixation rates in the bloom-forming cyanobacterium Nodularia spumigena. Biogeosciences. 2009;6:1865–1875. doi: 10.5194/bg-6-1865-2009. [DOI] [Google Scholar]
- Doney SC, Mahowald N, Lima L, Feely RA, Mackenzie FT, Lamarque JF, Rasch PJ. Impact of anthropogenic atmospheric nitrogen and sulfur deposition on ocean acidification and the inorganic carbon system. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14580–14585. doi: 10.1073/pnas.0702218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: The other CO2 problem. Annual Review of Marine Science. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- Doney S, Ruckelshaus M, Duffy E, Barry J, Chan F, English C, Galindo H, Grebmeier J, et al. Climate change impacts on marine ecosystems. Annual Review of Marine Science. 2012;4:1–27. doi: 10.1146/annurev-marine-041911-111611. [DOI] [PubMed] [Google Scholar]
- Eklöf, J., Alsterberg, C., Havenhand, J., Sundbäck, K., Wood, H. and Gamfeldt, L. 2012. Experimental climate change weakens the insurance effect of biodiversity. Ecology Letters. doi:10.1111/j.1461-0248.2012.01810.x. [DOI] [PubMed]
- Findlay HS, Burrows MT, Kendall MA, Spicer JI, Widdicombe S. Can ocean acidification affect population dynamics of the barnacle Semibalanus balanoides at its southern range edge? Ecology. 2010;91:2931–2940. doi: 10.1890/09-1987.1. [DOI] [PubMed] [Google Scholar]
- Findlay HS, Kendall MA, Spicer JI, Widdicombe S. Relative influences of ocean acidification and temperature on intertidal barnacle post-larvae at the northern edge of their geographic distribution. Estuarine, Coastal and Shelf Science. 2010;86:675–682. doi: 10.1016/j.ecss.2009.11.036. [DOI] [Google Scholar]
- Franke A, Clemmesen C. Effect of ocean acidification on early life stages of Atlantic herring (Clupea harengus L.) Biogeosciences Discussions. 2011;8:7097–7126. doi: 10.5194/bgd-8-7097-2011. [DOI] [Google Scholar]
- Frommel AY, Stiebens V, Clemmesen C, Havenhand J. Effect of ocean acidification on marine fish sperm (Baltic cod: Gadus morhua) Biogeosciences. 2010;7:3915–3919. doi: 10.5194/bg-7-3915-2010. [DOI] [Google Scholar]
- Frommel AY, Maneja R, Lowe D, Malzahn AM, Geffen AJ, Folkvord A, Piatkowski U, Clemmesen C. Ocean acidification effects on larvae of a commercially important fish species, Atlantic cod (Gadhus morhua) Nature Climate Change. 2012;2:42–46. doi: 10.1038/nclimate1324. [DOI] [Google Scholar]
- Fu FX, Place AR, Garcia NS, Hutchins DA. CO(2) and phosphate availability control the toxicity of the harmful bloom dinoflagellate Karlodinium veneficum. Aquatic Microbial Ecology. 2010;59:55–65. doi: 10.3354/ame01396. [DOI] [Google Scholar]
- Gaylord B, Hill TM, Sanford E, Lenz EA, Jacobs LA, Sato KN, Russell AD, Hettinger A. Functional impacts of ocean acidification in an ecologically critical foundation species. Journal of Experimental Biology. 2011;214:2586–2594. doi: 10.1242/jeb.055939. [DOI] [PubMed] [Google Scholar]
- Gazeau, F., C. Quiblier, J.M. Jansen, J.P. Gattuso, J.J. Middelburg, and C.H.R. Heip. 2007. Impact of elevated CO2 on shellfish calcification. Geophysical Research Letters 34. doi:10.1029/2006GL028554.
- Gazeau F, Gattuso JP, Dawber C, Pronker AE, Peene F, Peene J, Heip CHR, Middelburg JJ. Effect of ocean acidification on the early life stages of the blue mussel Mytilus edulis. Biogeosciences. 2010;7:2051–2060. doi: 10.5194/bg-7-2051-2010. [DOI] [Google Scholar]
- Giordano M, Beardall J, Raven JA. CO(2) concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Annual Review of Plant Biology. 2005;56:99–131. doi: 10.1146/annurev.arplant.56.032604.144052. [DOI] [PubMed] [Google Scholar]
- Haddock SHD. Reconsidering evidence for potential climate-related increases in jellyfish. Limnology and Oceanography. 2008;53:2759–2762. [Google Scholar]
- Harvey CJ, Cox SP, Essington TE, Hansson S, Kitchell JF. An ecosystem model of food web and fisheries interactions in the Baltic Sea. ICES Journal of Marine Science. 2003;60:939–950. doi: 10.1016/S1054-3139(03)00098-5. [DOI] [Google Scholar]
- Heikinheimo O. Interactions between cod, herring and sprat in the changing environment of the Baltic Sea: A dynamic model analysis. Ecological Modelling. 2011;222:1731–1742. doi: 10.1016/j.ecolmodel.2011.03.005. [DOI] [Google Scholar]
- Hepburn CD, Pritchard DW, Cornwall CE, McLeod RJ, Beardall J, Raven JA, Hurd CL. Diversity of carbon use strategies in a kelp forest community: Implications for a high CO(2) ocean. Global Change Biology. 2011;17:2488–2497. doi: 10.1111/j.1365-2486.2011.02411.x. [DOI] [Google Scholar]
- Hofmann, G.E., J.E. Smith, K.S. Johnson, U. Send, L.A. Levin, F. Micheli, A. Paytan, N.N. Price, et al. 2011. High-frequency dynamics of ocean pH: A multi-ecosystem comparison. PLoS One 6. doi:10.1371/journal.pone.0028983. [DOI] [PMC free article] [PubMed]
- Hopkinson BM, Dupont CL, Allen AE, Morel FMM. Efficiency of the CO(2)- of diatoms. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3830–3837. doi: 10.1073/pnas.1018062108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC. 2007. Climate change 2007: The physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change, Cambridge: CUP.
- Karlberg, M., and A. Wulff. 2012. Climate change effects on filamentous cyanobacteria—two multifactorial case studies on ocean acidification, temperature and salinity. Marine Biology.
- Kordas RL, Harley CDG, O’Connor MI. Community ecology in a warming world: The influence of temperature on interspecific interactions in marine systems. Journal of Experimental Marine Biology and Ecology. 2011;400:218–226. doi: 10.1016/j.jembe.2011.02.029. [DOI] [Google Scholar]
- Kroeker KJ, Kordas RL, Crim RN, Singh GG. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecology Letters. 2010;13:1419–1434. doi: 10.1111/j.1461-0248.2010.01518.x. [DOI] [PubMed] [Google Scholar]
- Kurihara H, Ishimatsu A. Effects of high CO2 seawater on the copepod (Acartia tsuensis) through all life stages and subsequent generations. Marine Pollution Bulletin. 2008;56:1086–1090. doi: 10.1016/j.marpolbul.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Kurihara H, Shimode S, Shirayama Y. Sub-lethal effects of elevated concentration of CO2 on planktonic copepods and sea urchins. Journal of Oceanography. 2004;60:743–750. doi: 10.1007/s10872-004-5766-x. [DOI] [Google Scholar]
- Kurihara H, Asai T, Kato S, Ishimatsu A. Effects of elevated pCO(2) on early development in the mussel Mytilus galloprovincialis. Aquatic Biology. 2009;4:225–233. doi: 10.3354/ab00109. [DOI] [Google Scholar]
- Mayor DJ, Matthews C, Cook K, Zuur AF, Hay S. CO2-induced acidification affects hatching success in Calanus finmarchicus. Marine Ecology-Progress Series. 2007;350:97. doi: 10.3354/meps07142. [DOI] [Google Scholar]
- Melzner, F., P. Stange, K. Trubenbach, J. Thomsen, L. Casties, U. Panknin, S.N. Gorb, and M.A. Gutowska. 2011. Food supply and seawater pCO(2) impact calcification and internal shell dissolution in the blue mussel Mytilus edulis. Plos One 6. doi:10.1371/journal.pone.0024223. [DOI] [PMC free article] [PubMed]
- Melzner F, Gobel S, Langenbuch M, Gutowska MA, Pörtner HO, Lucassen M. Swimming performance in Atlantic Cod (Gadus morhua) following long-term (4–12 months) acclimation to elevated seawater P(CO2) Aquatic Toxicology. 2009;92:30–37. doi: 10.1016/j.aquatox.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Melzner F, Gutowska MA, Langenbuch M, Dupont S, Lucassen M, Thorndyke MC, Bleich M, Pörtner HO. Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences. 2009;6:2313–2331. doi: 10.5194/bg-6-2313-2009. [DOI] [Google Scholar]
- Niiranen, S., M.T. Tomczak, O. Hjerne, and T. Blenckner. 2012. Uncertainties in a Baltic Sea food-web model reveal challenges for future projections. AMBIO. doi:10.1007/s13280-012-0324-z. [DOI] [PMC free article] [PubMed]
- Omstedt A, Gustafsson E, Wesslander K. Modelling the uptake and release of carbon dioxide in the Baltic Sea surface water. Continental Shelf Research. 2009;29:870–885. doi: 10.1016/j.csr.2009.01.006. [DOI] [Google Scholar]
- Omstedt A, Edman M, Anderson LG, Laudon H. Factors influencing the acid-base (pH) balance in the Baltic Sea: A sensitivity analysis. Tellus Series B-Chemical and Physical Meteorology. 2010;62:280–295. doi: 10.1111/j.1600-0889.2010.00463.x. [DOI] [Google Scholar]
- Palacios SL, Zimmerman RC. Response of eelgrass Zostera marina to CO2 enrichment: Possible impacts of climate change and potential for remediation of coastal habitats. Marine Ecology-Progress Series. 2007;344:1–13. doi: 10.3354/meps07084. [DOI] [Google Scholar]
- Ploug H. Cyanobacterial surface blooms formed by Aphanizomenon sp. and Nodularia spumigena in the Baltic Sea: Small-scale fluxes, pH, and oxygen microenvironments. Limnology and Oceanography. 2008;53:914–921. doi: 10.4319/lo.2008.53.3.0914. [DOI] [Google Scholar]
- Pörtner HO. Ecosystem effects of ocean acidification in times of ocean warming: A physiologist’s view. Marine Ecology-Progress Series. 2008;373:203–217. doi: 10.3354/meps07768. [DOI] [Google Scholar]
- Pörtner HO, Langenbuch M, Reipschlager A. Biological impact of elevated ocean CO2 concentrations: Lessons from animal physiology and earth history. Journal of Oceanography. 2004;60:705–718. doi: 10.1007/s10872-004-5763-0. [DOI] [Google Scholar]
- Riebesell U, Schulz KG, Bellerby RGJ, Botros M, Fritsche P, Meyerhofer M, Neill C, Nondal G, et al. Enhanced biological carbon consumption in a high CO2 ocean. Nature. 2007;450:545–548. doi: 10.1038/nature06267. [DOI] [PubMed] [Google Scholar]
- SMHI. 2011. Swedish Oceanographic Data Centre. http://www.smhi.se/oceanografi/oce_info_data/SODC/download_en.htm. Accessed 15 March 2011.
- Sparholt, H. 1991. Multispecies assessment of Baltic fish stocks. Multispecies models relevant to management of living resources. ICES Marine Science Symposium 193: 64–79.
- Sunday, J.M., R.N. Crim, C.D.G. Harley, and M.W. Hart. 2011. Quantifying rates of evolutionary adaptation in response to ocean acidification. Plos One 6. doi:10.1371/journal.pone.0022881. [DOI] [PMC free article] [PubMed]
- Tedengren M, Kautsky N. Comparative stress response to diesel oil and salinity changes of the blue mussel, Mytilus edulis from the Baltic and North seas. Ophelia. 1987;28:1–9. doi: 10.1080/00785326.1987.10430800. [DOI] [Google Scholar]
- Thomsen J, Gutowska MA, Saphorster J, Heinemann A, Trubenbach K, Fietzke J, Hiebenthal C, Eisenhauer A, et al. Calcifying invertebrates succeed in a naturally CO(2)-rich coastal habitat but are threatened by high levels of future acidification. Biogeosciences. 2010;7:3879–3891. doi: 10.5194/bg-7-3879-2010. [DOI] [Google Scholar]
- Tomczak MT, Niiranen S, Hjerne O, Blenckner T. Ecosystem flow dynamics in the Baltic Proper—using a multi-trophic dataset as a basis for food-web modelling. Ecological Modelling. 2012;230:123–147. doi: 10.1016/j.ecolmodel.2011.12.014. [DOI] [Google Scholar]
- Tyrrell T, Schneider B, Charalampopoulou A, Riebesell U. Coccolithophores and calcite saturation state in the Baltic and Black Seas. Biogeosciences. 2008;5:485–494. doi: 10.5194/bg-5-485-2008. [DOI] [Google Scholar]
- Walther K, Anger K, Portner HO. Effects of ocean acidification and warming on the larval development of the spider crab Hyas araneus from different latitudes (54 degrees vs. 79 degrees N) Marine Ecology-Progress Series. 2010;417:159–170. doi: 10.3354/meps08807. [DOI] [Google Scholar]
- Walther K, Sartoris FJ, Portner H. Impacts of temperature and acidification on larval calcium incorporation of the spider crab Hyas araneus from different latitudes (54 degrees vs. 79 degrees N) Marine Biology. 2011;158:2043–2053. doi: 10.1007/s00227-011-1711-x. [DOI] [Google Scholar]
- Westerbom M, Mustonen O, Kilpi M. Distribution of a marginal population of Mytilus edulis: Responses to biotic and abiotic processes at different spatial scales. Marine Biology. 2008;153:1153–1164. doi: 10.1007/s00227-007-0886-7. [DOI] [Google Scholar]
- Wootton JT, Pfister CA, Forester JD. Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18848–18853. doi: 10.1073/pnas.0810079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman RC, Kohrs DG, Steller DL, Alberte RS. Impacts of CO2 enrichment on productivity and light requirements of eelgrass. Plant Physiology. 1997;115:599–607. doi: 10.1104/pp.115.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]