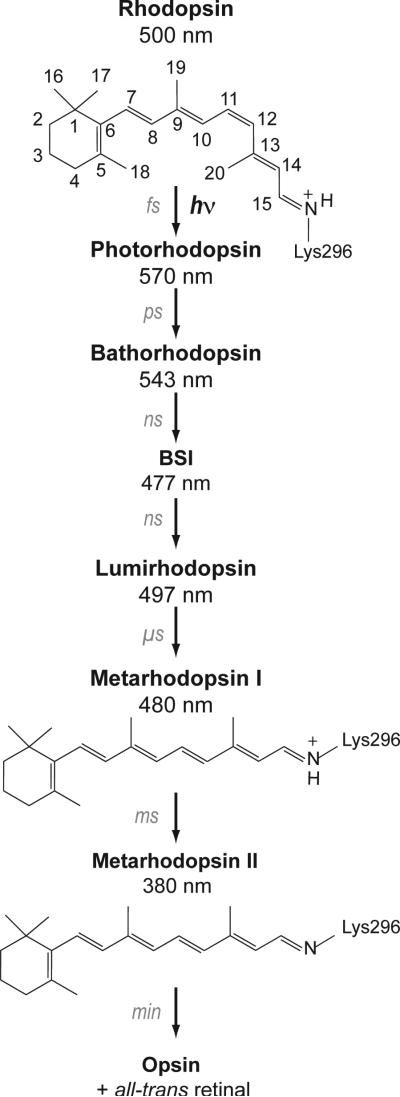
Figure 2.
Photoreaction of rhodopsin. Structures of the 11-cis and all-trans retinal chromophores and the photoreaction intermediates of rhodopsin are shown. Absorption of light results in 11-cis to all-trans isomerization of the retinal. The retinal-protein complex subsequently relaxes thermally through a series of spectrally well-defined intermediates: Photorhodopsin, Bathorhodopsin, the Blue Shifted Intermediate (BSI), Lumirhodopsin, and Meta I. Deprotonation of the retinal Schiff base nitrogen occurs in the formation of the Meta II intermediate. Meta I exists in a pH- and temperature-dependent equilibrium with the active Meta II intermediate.89 High pH and low temperature favor Meta I. Evidence for substantial structural changes in Meta I comes from time resolved absorbance measurements showing that the transition to Lumirhodopsin is the last step in the photoreaction sequence that does not exhibit differences between lipid and detergent environments.90 In addition, Meta I is the first intermediate that cannot be fully photo-converted back to rhodopsin.91