Abstract
c-Met represents an important emerging therapeutic target in cancer. Here, we demonstrate the mechanism by which c-Met tyrosine kinase inhibition inhibits tumor growth in a highly invasive Asian-prevalent head and neck cancer, nasopharyngeal cancer (NPC). c-Met tyrosine kinase inhibitors (TKIs; AM7 and c-Met TKI tool compound SU11274) downregulated c-Met phosphorylation resulting in markedly inhibited growth and invasion of NPC cells. Strikingly, inhibition of c-Met resulted in marked downregulation of TIGAR (TP53-induced Glycolysis and Apoptosis Regulator) and subsequent depletion of intracellular NADPH. Importantly, overexpression of TIGAR ameliorated the effects of c-Met kinase inhibition, confirming the importance of TIGAR downregulation in growth inhibition induced by c-Met TKI. The effects of c-Met inhibition on TIGAR and NADPH levels were observed with two different c-Met TKIs (AM7 and SU11274) and with multiple cell lines. As NADPH provides a crucial reducing power required for cell survival and proliferation, our findings represent a novel mechanistic action of c-Met TKI, which may represent a key effect of c-Met kinase inhibition. Our data provides the first evidence linking c-Met, TIGAR and NADPH regulation in human cancer cells suggesting that inhibition of a tyrosine kinase/TIGAR/NADPH cascade may have therapeutic applicability in human cancers.
Keywords: c-Met tyrosine kinase inhibitor, TIGAR, NADPH
Introduction
c-Met is rapidly emerging as an important target for cancer therapy. Overexpression of c-Met is associated with poor patient survival or metastasis in numerous human malignancies (Comoglio et al., 2008). Cumulative in vitro and in vivo evidences demonstrates that c-Met targeting can effectively attenuate tumor growth, invasion and metastasis (Comoglio et al., 2008), however, the mechanisms remain to be fully elucidated. Phase I and II human clinical trials of multiple c-Met targeting approaches are currently underway, including monoclonal antibodies against HGF (hepatocyte growth factor, the ligand of c-Met) and small molecule tyrosine kinase inhibitors (TKIs) against c-Met. Although the results of clinical trials are yet to be revealed, many of the c-Met TKIs demonstrated promising preclinical efficacy in inhibiting tumor growth, invasion, metastasis, as well as angiogenesis (Comoglio et al., 2008) resulting in optimism for the approach.
Gain-of-function aberrations affecting c-Met, which causes inappropriate activation of c-Met, is an oncogenic event (Cooper et al., 1984; Wang et al., 2001). In various cancers, gain-of-function of c-Met occurs via genetic alterations (e.g. gene amplification or rearrangement, activating mutation), overexpression (by transcriptional upregulation), as well as upregulation of its ligand, HGF (Comoglio et al., 2008; Nakamura et al., 2008). Although the incidence of activating c-Met mutation or gene rearrangement is relatively low in human cancers, overexpression of c-Met protein (either via transcriptional activation or gene amplification) is observed in >50–80% of a wide variety of cancers (Comoglio et al., 2008). Recently, Wang et al showed that overexpression of wildtype c-Met in hepatocytes was sufficient to cause liver cancer (Wang et al., 2001), providing support for c-Met overexpression being a causal event in cancer. Targeting of aberrant c-Met activation, either overexpression of wildtype or activating mutants of c-Met, has demonstrated therapeutic efficacy in diverse cancer models (Stabile et al., 2004; Comoglio et al., 2008).
In view of this, several novel and versatile c-Met inhibitors with potent inhibitory activities against both overexpressed wildtype as well as activating mutants of c-Met have been developed (Bellon et al., 2008). AM7 is a recently developed c-Met inhibitor with a distinct binding mode to c-Met as compared to classical c-Met TKI “tool compound” (e.g. SU11274) (Bellon et al., 2008). X-ray crystallography revealed that AM7 binds to the kinase linker region and extends into a hydrophobic binding site of c-Met, thus allowing interaction with and subsequent inhibition of both wildtype and mutated c-Met (Bellon et al., 2008). Preclinical studies are underway to determine the therapeutic efficacy of this new class of c-Met TKI.
Nasopharyngeal cancer (NPC) is a highly metastatic head and neck cancer prevalent in Asia, with an annual incidence of 15-30/100,000cases/year (comparable to that of pancreatic cancer in the US)(Yu and Yuan, 2002; Anderson et al., 2006). Over 60–70% of NPC patients have advanced disease (Stage IIb or IV) at the time of diagnosis. c-Met overexpression correlates with poor survival and metastasis in NPC (Qian et al., 2002), suggesting that c-Met may be an etiological agent and therapeutic target in this disease. In this study, we demonstrate that inhibition of c-Met (by AM7 and a c-Met TKI tool compound SU11274) blocks growth and invasion of NPC cells. Strikingly, c-Met inhibition decreased p53 and downregulated TIGAR, resulting in a significant reduction of cellular NADPH levels. As NADPH provides reducing power required for cell survival and proliferation, this may represent a novel mechanistic target of c-Met TKI. Indeed, overexpression of TIGAR rescued NPC cells from both AM7- and SU11274-mediated growth inhibition, demonstrating the mechanistic importance of TIGAR down-modulation. The effects of c-Met inhibition on TIGAR and NADPH levels were generalizable with both c-Met TKIs (AM7 and SU11274) demonstrating activity across multiple cell lines. Our findings not only demonstrated the therapeutic potential of c-Met targeting in NPC, but also revealed novel effects of c-Met targeting on NADPH regulation in cancer cells via TIGAR. Our study provides the first evidence linking c-Met, TIGAR and NADPH regulation in cancer cells, suggesting that inhibition of a tyrosine kinase/TIGAR/NADPH cascade may have therapeutic applicability in cancer treatment.
Results and Discussions
AM7 inhibits NPC cell proliferation
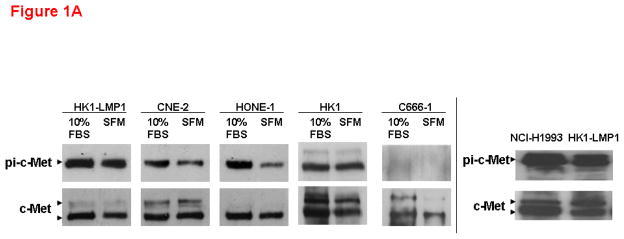
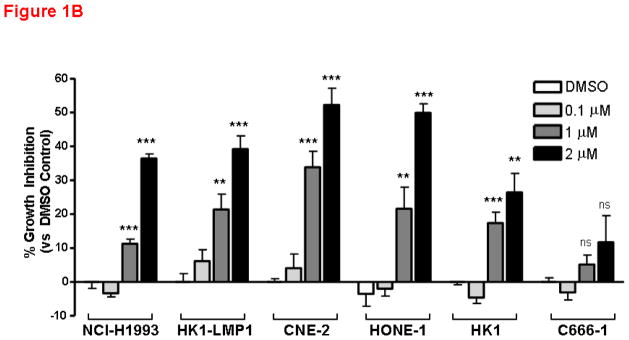
c-Met is frequently overexpressed in NPC patients, as well as NPC cell lines(Qian et al., 2002). As shown in Figure 1A, most NPC cell lines, except C666-1, expressed high levels of total c-Met and phoshorylated c-Met, which serves as a surrogate for activated c-Met, under basal (10% FBS) and serum-starved conditions, albeit frequently with lower levels in serum-starved cells. The effect of AM7 on NPC cell growth was examined. A well-known c-Met TKI-sensitive cell line, NCI-H1993 (with c-Met gene amplification) was also included as a positive control. As shown in Figure 1B, inhibition of c-Met with AM7 induced a dose-dependent growth inhibition in both NCI-H1993 and 4/5 NPC cell lines tested. Cumulative results demonstrated that AM7-mediated growth inhibition of NPC cell lines was comparable to that of NCI-H1993 at 2μM concentration as early as 48h (n≥9–21; p<0.01**, p<0.0001***), except for C666-1 (n≥12; p>0.05, ns). It is important to note that Het-1A, a normal esophageal epithelial control cell line (ATCC, USA) has undetectable levels of c-Met (by Western blotting of up to 50μg total protein, Supplementary Figure 1A), was not sensitive to AM7 (Supplementary Figure 1B). Our data indicate that inhibition of c-Met with AM7, a new c-Met TKI, can specifically abrogate growth of NPC cells with c-Met overexpression/activation.
Figure 1. c-Met inhibitor elicits substantial growth inhibition on NPC cell lines.
(A) c-Met expression and activation in NPC cells. All human NPC cell lines were maintained as previously described (Horikawa et al., 2001; Tulasne and Foveau, 2008). Basal levels of c-Met and activated c-Met in 10% FBS or under serum-starvation for 24h were shown for 5 NPC cell lines. A c-Met-TKI sensitive cell line, NCI-H1993 (non-small cell lung cancer cell line with c-Met gene amplification; purchased from ATCC, USA) was included as positive control for drug sensitivity. Phospho-c-Met was detected by immunoprecipitation as previously described (Zhang et al., 2006; Wong et al., 2009). Phosphorylated c-Met was immunopreciptated with c-Met antibody (C-12) and probed with phospho-Tyr99 antibody (both from Santa Cruz Biotechnology, USA). Right: HK1-LMP1 showed comparable basal level of phospho-c-Met to that of NCI-H1993. (B) Dose-dependent growth inhibition of NPC cell lines by AM7. AM7 was kindly provided by Amgen Inc., USA. Both NCI-H1993 (positive control cell line) and NPC cell lines were treated with increasing doses of AM7 (0.1, 1, 2μM) or DMSO (vehicle) for 48h. Percentage growth inhibition (vs DMSO) was determined in triplicate for each independent experiment (mean ± SEM) by MTT assay as previously described (Lui et al., 2007). Bar graph shows cumulative results of ≥3–7 independent experiments in each cell line (n≥9–21; p≤0.0001***; p≤0.005***).
Although AM7 has the ability to target both wildtype and mutated c-Met, the sensitivity of NPC cell lines towards AM7 was not due to mutation of the c-Met tyrosine kinase (TK). Mutational analysis of the c-Met tyrosine kinase domain (nucleotide 3232–4011 of coding sequence, which represents the most common site of activating mutations in c-Met) of all five NPC cell lines revealed that c-Met TK domain mutation was uncommon in NPC cells with all detectable nucleotide changes in the TK domain being “silent mutations”, conferring no change in amino acid sequence (Supplementary Table 1). Although it is unclear whether other activating c-Met mutations occur in NPC patients, our results demonstrated that inhibition of c-Met may demonstrate therapeutic efficacy even in the absence of activating c-Met mutation. Interestingly, the NPC cell lines with comparable AM7 sensitivity to NCI-H1993 (known to be c-Met TKI-responsive due to c-Met gene amplification) do not carry any c-Met gene amplification as analyzed by FISH analysis (Supplementary Figure 1C).
AM7 inhibits c-Met signaling and reduced invasiveness of NPC cells
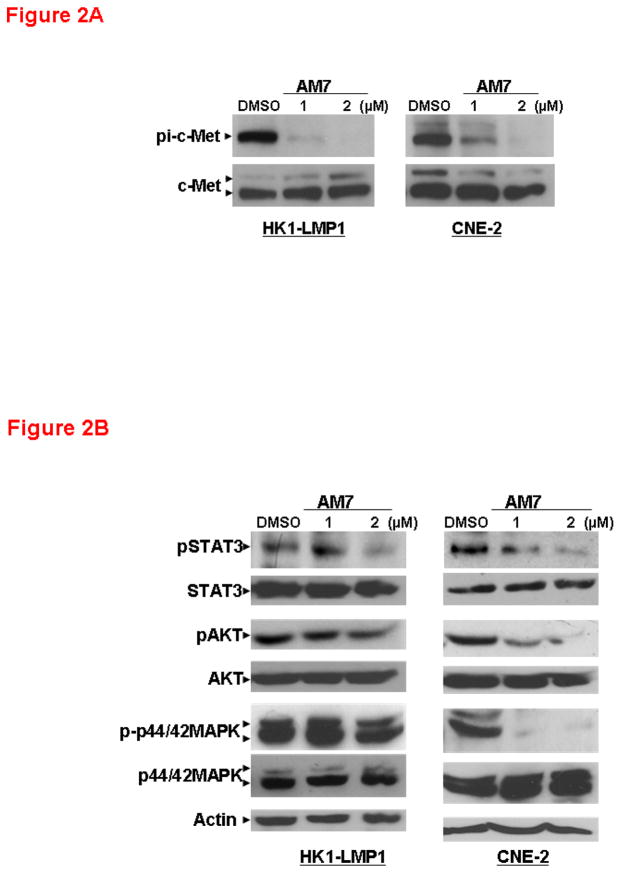
Next, we investigated the mechanistic effects of AM7 on c-Met signaling in two representative NPC cell lines with that were sensitive to AM7 (namely HK1-LMP1, an EBV-associated NPC cell line expressing the EBV oncoprotein, the latent membrane protein 1 [LMP1]; and CNE-2 with poorly differentiated NPC origin). In both cell lines, AM7 exhibited potent inhibition of c-Met phosphorylation as early as 2h, while total c-Met level remained unchanged (Figure 2A). Blockade of c-Met signaling by AM7 also led to inhibition of several major downstream signaling pathways including STAT3 and p44/42 MAPK. In both HK1-LMP1 and CNE-2, AM7 induced a dose-dependent inhibition of STAT3 phosphorylation (Tyr705) and p44/42 MAPK activation as early as 2h (Figure 2B), which agreed with previous time courses of c-Met inhibition (Ma et al., 2007). Similar results were observed in another LMP1-expressing and AM7-sensitive NPC cell line, CNE-2-LMP1 (Supplementary Figure 2). The effect of AM7 on AKT pathway was more significant in CNE-2 than in the two LMP1-expressing NPC cell lines, HK1-LMP1 (Figure 2B) and CNE-2-LMP1 (Supplementary Figure 2B). Although our data suggests a potential involvement of different downstream signaling effectors of c-Met in NPC cells with or without LMP1 expression, AM7 efficiently inhibited signaling downstream of c-Met in several NPC cell lines.
Figure 2. Inhibition of c-Met phosphorylation and downstream signaling in NPC cells by AM7.
(A) Complete attenuation of c-Met phosphorylation by AM7 (by Western blotting). HK1-LMP1 and CNE-2 cells were treated with AM7 or DMSO in complete medium and were harvested after 2h. (B) AM7 reduced the expression levels of c-Met downstream signaling molecules as early as 2h. Expression of total and phospho-STAT3, AKT and p44/42 MAPK were shown (See Supplementary Information for antibody sources). Similar results were obtained in 3 independent experiments.
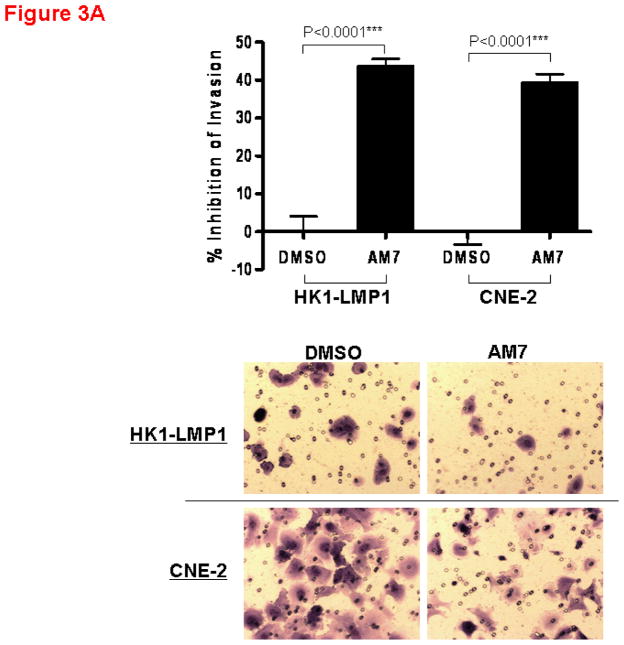
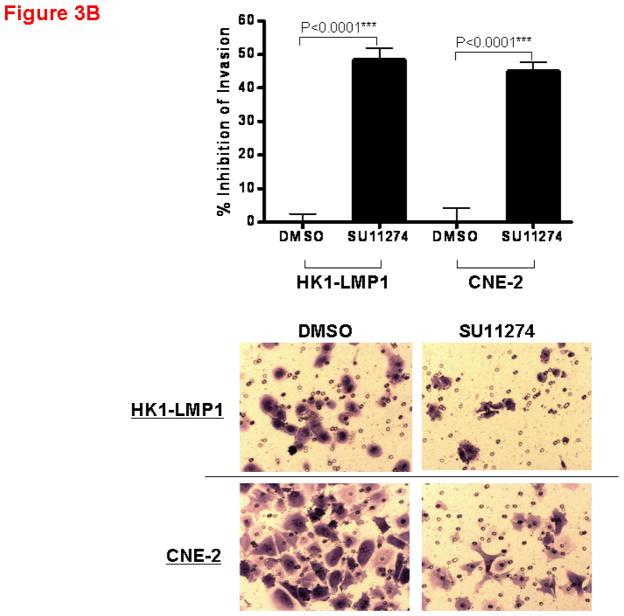
The effect of AM7 on the invasiveness of NPC cells was also examined as c-Met has been clinically implicated in NPC progression (Horikawa et al., 2001). As shown in Figure 3A, AM7 (2μM) significantly inhibited cellular invasion (by Matrigel invasion assay) in both cell lines with the invasiveness of HK1-LMP1 and CNE-2 being reduced by AM7 by 44% and 39% respectively, when compared to DMSO control. The c-Met TKI, SU11274 was also effective in inhibiting cellular invasiveness (Figure 3B). Our results suggest that inhibition of c-Met by both AM7 and SU11274 blocks both NPC cell invasion and proliferation.
Figure 3. Inhibition of NPC cell invasion through the Matrigel by AM7 and SU11274. Bar graphs showing percent inhibition of cellular invasion through Matrigel invasion chamber by AM7 vs. DMSO (A) or SU11274 vs. DMSO (B) at 24h for both HK1-LMP1 and CNE-2 cells.
Both assays were performed as previously described (Lui et al., 2009a; Wong et al., 2009). HK1-LMP1 (1.0×105) and CNE-2 (5.0×104) cells in serum-free medium were incubated with AM7 (2μM) or SU11274 (5μM) or DMSO in the Matrigel gel invasion chambers for 24h. Invaded cells were fixed, stained and counted. For each independent experiment, quantitative results were calculated from 10 random fields (n=10, p<0.0001***) and photographed. Graphs showed the cumulative results of 3 independent experiments.
AM7 and SU11274 reduce TIGAR expression and cellular NADPH level in NPC cells
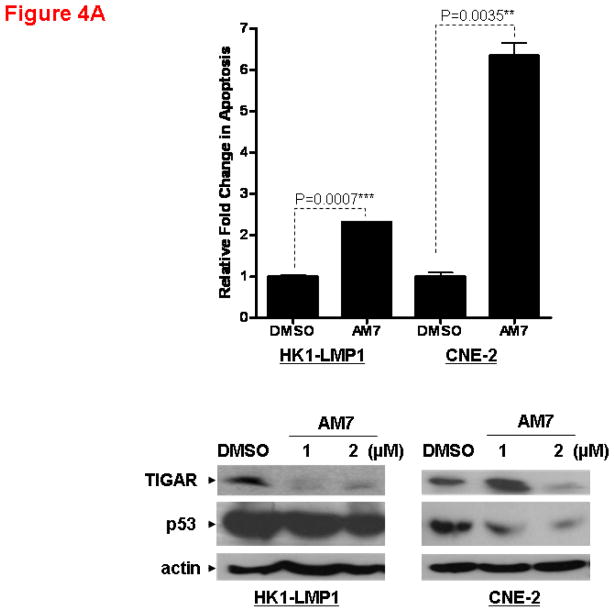
Since c-Met is a key regulator of cell survival and apoptosis(Tulasne and Foveau, 2008), we investigated the effects of AM7 on apoptosis. Using a quantitative Cell Death Detection ELISA assay measuring DNA fragmentation (Roche Diagnostics, Germany), we demonstrated that AM7 was able to induce apoptosis in NPC cell lines as early as 48h (Figure 4A).
Figure 4. c-Met inhibitors, AM7 and SU11274, reduce TIGAR expression and cellular NADPH level, leading to apoptosis.
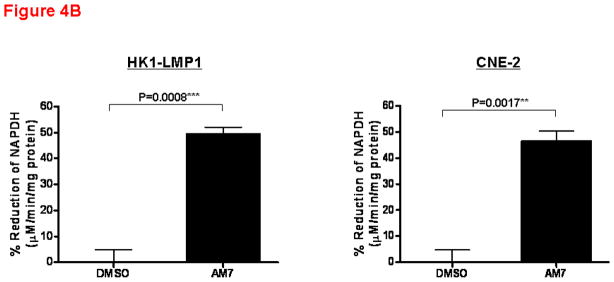
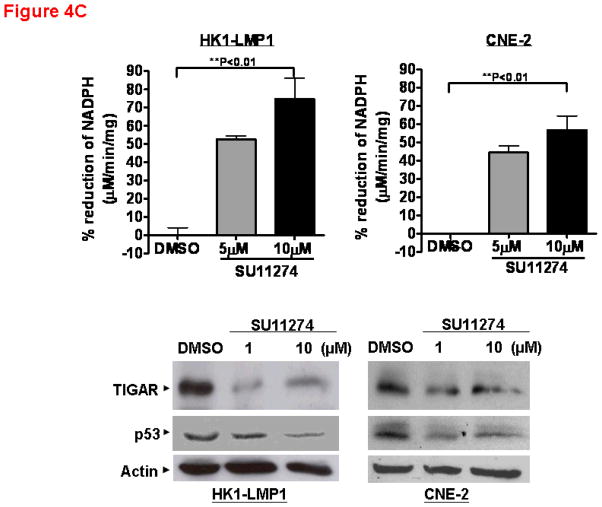
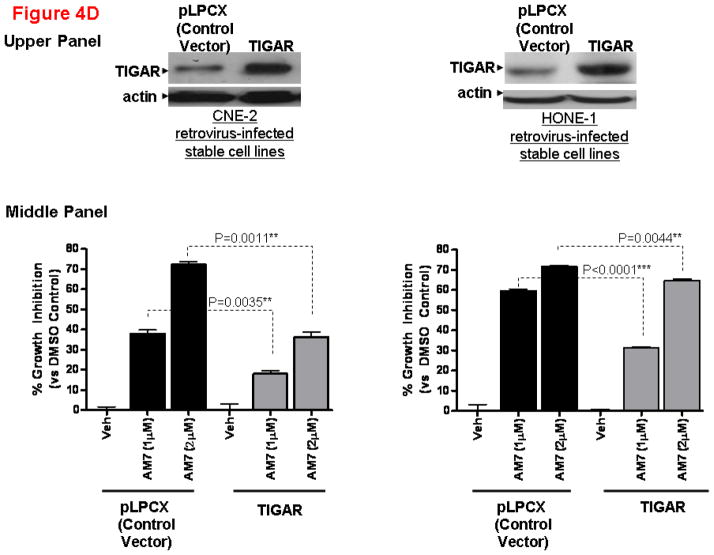
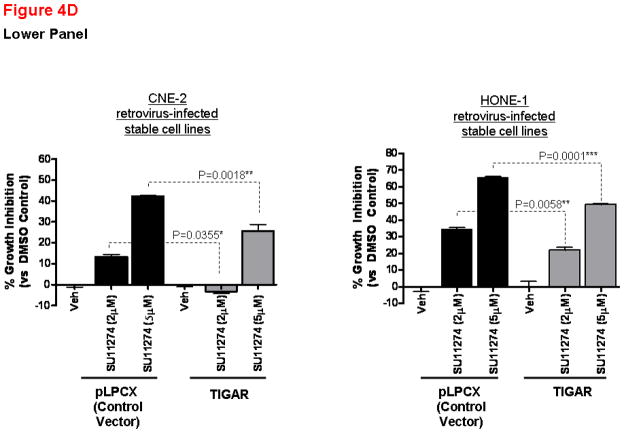
(A) Apoptosis of NPC cells is associated with p53 and TIGAR downregulation. Cell death by apoptosis (as measured by DNA fragmentation) was quantitatively determined using the Cell Death Detection ELISAPLUS kit (Roche Diagnostics, Germany) according to the manufacturer’s instruction. Cells were treated with AM7 (2μM) or SU11274 (5μM) or DMSO for 48h and harvested for cell death quantification. Fold change in apoptosis relative to the respective DMSO control was presented for each cell line (mean ± SEM, n=3) and similar results were obtained in 3 independent experiments. Lower panel: Western blots showing the expression levels of TIGAR and p53 in HK1-LMP1 and CNE-2 cells at 48h of AM7 (2μM) or SU11274 (5μM) or DMSO treatment (See Supplementary Information for antibody sources). Similar results were obtained in 3 independent experiments. (B). AM7 induces dose-dependent reduction of intracellular NADPH in NPC cells. HK1-LMP1 and CNE-2 cells were treated with AM7 (2μM) or DMSO in complete medium for 48h. Cellular NADPH production was measured at 30min according to the manufacturer’s instruction (See details in Supplementary Information) and normalized to total protein as μM/min/mg total protein. Percentage reduction of NADPH was calculated with reference to DMSO. Graph show percentage reduction of NADPH level of AM7-treated cells vs DMSO-treated cells (mean ± SEM, n=3). Similar results were obtained in 3 independent experiments. (C) A model c-Met TKI, SU11274 downregulates TIGAR and NADPH levels in NPC cells. HK1-LMP1 and CNE-2 cells were treated with SU11274 or DMSO in complete medium for 48h. Cellular NADPH production was determined as above. The expression levels of TIGAR and p53 were also shown. (D) Overexpression of TIGAR rescues NPC cells from both AM7- and SU11274-mediated growth inhibition. Infective retroviruses expressing human TIGAR gene (original gene construct of human TIGAR was obtained from Origene, USA) or the control vector (pLPCX control vector for retroviral gene delivery; Clontech, USA) were generated. Retrovirus infection into NPC cells (CNE-2 and HONE-1) was performed, followed by puromycin selection (400ng/ml) as previously described (Lui et al., 2009b). Upon confirmation of TIGAR overexpression (by Western blotting for TIGAR; Upper Panel) in these stable cell lines, puromycin selection was removed periodically. Retrovirus-infected stable cell lines from CNE-2 and HONE-1 origin were treated with AM7 (2μM) or SU11274 (5μM) or DMSO in 3% FBS for 72h. Effects of TIGAR overexpression on AM7- or SU11274-induced growth inhibition (as % growth inhibition vs DMSO control) was assayed by MTT assay and presented in the middle and lower panels, respectively. Similar results were obtained in 3 independent experiments.
Unexpectedly, a newly described regulator of apoptosis and glycolysis, TIGAR (TP53-induced Glycolysis and Apoptosis Regulator)(Bensaad et al., 2006), was markedly reduced by AM7 in both cell lines. The observed reduction of TIGAR expression was consistent with reduced expression of its upstream regulator, p53 (Figure 4A).
TIGAR has recently been found to be a dual regulator of apoptosis and glycolysis. It has been postulated that TIGAR may inhibit apoptosis via regulation of cellular NADPH levels (through modulation of the pentose phosphate pathway) (Bensaad et al., 2006). NADPH provides the major reducing power in mammalian cells, which is critical for protection of cells from oxidative stress/damage, as well as for reductive biosynthesis of important biomolecules, including DNA, RNA, fatty acids, and cholesterol. Rapidly proliferating cells thus require NADPH for normal function, proliferation and survival. Our finding that c-Met blockade by AM7 downregulated TIGAR expression in NPC cells suggested a potential effect of this c-Met TKI on cellular NADPH production. As shown in Figure 4B, AM7 reduced intracellular NADPH in both HK1-LMP1 and CNE-2 cells by 47–49%, when compared to DMSO control. Similar results were observed with SU11274 indicating that the effects are generalizable to multiple modes of c-Met inhibition (Figure 4C). This provides the first demonstration in human cancer cells that c-Met inhibition could result in significant reduction of cellular NADPH, the key reducing power in mammalian cells. This novel finding was consistent with the demonstration that specific downregulation of TIGAR by siRNA induced cancer cell death by altering the pentose phosphate pathway (Bensaad et al., 2006), which is the major pathway regulating the production of cellular NADPH. Our data implicates a potential link between c-Met signaling and NADPH regulation in cancer cells.
Since AM7-induced TIGAR downregulation was accompanied by reduction of NADPH levels in NPC cells, and rapidly proliferating cells require NADPH for proliferation and survival, we hypothesized that TIGAR downregulation might contribute to AM7-induced growth inhibition of NPC cells. We performed rescue experiments to determine if overexpression of TIGAR could reverse growth inhibition in NPC cells induced by c-Met TKIs. Using retrovirus infection, we generated stable cell lines expressing TIGAR protein (human TIGAR gene construct was purchased from Origene, USA) (Figure 4D). In comparison with the vector control stable cell lines (pLPCX vector), TIGAR-expression in the stable cell lines was able to reduce the growth inhibitory effects of AM7 (at both 1 and 2μM) at 72h. Similarly, TIGAR-overexpression was also able to reduce SU11274-mediated NPC cell growth inhibition (at both 2 and 5μM doses) at 72h. In the faster-growing NPC cell line, HONE-1, a marked rescue from AM7 and SU11274-induced growth inhibition by TIGAR-overexpression was also observed even under serum-deprivation (Supplementary Figure 3). Our results established for the first time the mechanistic involvement of TIGAR in c-Met TKI-mediated growth inhibition in NPC cells.
In summary, our study not only demonstrates the therapeutic potential of c-Met targeting in NPC, but also provides the first demonstration of a novel antitumor mechanism of c-Met TKIs in inhibiting TIGAR expression and NADPH levels in human cancer cells. Our data provides the first evidence linking c-Met, TIGAR and NADPH regulation in human cancer cells, suggesting that inhibition of a tyrosine kinase/TIGAR/NADPH cascade may have therapeutic applicability in cancer treatment.
Supplementary Material
Acknowledgments
Financial Support: Research Grant Council, Hong Kong Government (471607) and Direct Grants for Research (2006.1.027; 2007.1.025; 2009.1.012) to VWYL. GBM is supported by Head and Neck SPORE P50 CA097007 and CCSG grant P30CA16672.
We are grateful for the research supports from the Research Grant Council, Hong Kong Government (471607) and Direct Grant for Research (2006.1.027; 2007.1.025; 2009.1.012) to VWYL; Head and Neck SPORE P50 CA097007 and CCSG grant P30CA16672 to GBM. We also thank Amgen, Inc. (USA) for the kind provision of the novel c-Met tyrosine kinase inhibitor, AM7.
Footnotes
The authors declare no conflict of interest.
Supplementary information is available at Oncogene website.
References
- Anderson KE, Mack T, Silverman DT. In: Cancer epidemiology and prevention. Schottenfeld D, Fraumeni JF Jr, editors. Oxford University Press; New York: 2006. pp. 721–762. [Google Scholar]
- Bellon SF, Kaplan-Lefko P, Yang Y, Zhang Y, Moriguchi J, Rex K, et al. c-Met inhibitors with novel binding mode show activity against several hereditary papillary renal cell carcinoma-related mutations. J Biol Chem. 2008;283:2675–2683. doi: 10.1074/jbc.M705774200. [DOI] [PubMed] [Google Scholar]
- Bensaad K, Cheung EC, Vousden KH. Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO J. 2009;28:3015–3026. doi: 10.1038/emboj.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008;7:504–516. doi: 10.1038/nrd2530. [DOI] [PubMed] [Google Scholar]
- Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984;311:29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- Horikawa T, Sheen TS, Takeshita H, Sato H, Furukawa M, Yoshizaki T. Induction of c-Met proto-oncogene by Epstein-Barr virus latent membrane protein-1 and the correlation with cervical lymph node metastasis of nasopharyngeal carcinoma. Am J Pathol. 2001;159:27–33. doi: 10.1016/S0002-9440(10)61669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang SX, Yamashita K, Yamamoto M, Piao CJ, Umezawa A, Saegusa M, et al. EGFR genetic heterogeneity of nonsmall cell lung cancers contributing to acquired gefitinib resistance. Int J Cancer. 2008;123(11):2480–6. doi: 10.1002/ijc.23868. [DOI] [PubMed] [Google Scholar]
- Lui VW, Boehm AL, Koppikar P, Leeman RJ, Johnson D, Ogagan M, et al. Antiproliferative mechanisms of a transcription factor decoy targeting signal transducer and activator of transcription (STAT) 3: the role of STAT1. Mol Pharmacol. 2007;71:1435–1443. doi: 10.1124/mol.106.032284. [DOI] [PubMed] [Google Scholar]
- Lui VW, Wong EY, Ho Y, Hong B, Wong SC, Tao Q, et al. STAT3 activation contributes directly to Epstein-Barr virus-mediated invasiveness of nasopharyngeal cancer cells in vitro. Int J Cancer. 2009a;125:1884–1893. doi: 10.1002/ijc.24567. [DOI] [PubMed] [Google Scholar]
- Lui VW, Yau DM, Wong EY, Ng YK, Lau CP, Ho Y, et al. Cucurbitacin I elicits anoikis sensitization, inhibits cellular invasion and in vivo tumor formation ability of nasopharyngeal carcinoma cells. Carcinogenesis. 2009b;30(12):2085–94. doi: 10.1093/carcin/bgp253. [DOI] [PubMed] [Google Scholar]
- Lui VW, Lau CP, Cheung CS, Ho K, Ng MH, Cheng SH, et al. An RNA-directed nucleoside anti-metabolite, 1-(3-C-ethynyl-beta-d-ribo-pentofuranosyl)cytosine (ECyd), elicits antitumor effect via TP53-induced Glycolysis and Apoptosis Regulator (TIGAR) downregulation. Biochem Pharmacol. 2010;79(12):1772–80. doi: 10.1016/j.bcp.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Ma PC, Tretiakova MS, Nallasura V, Jagadeeswaran R, Husain AN, Salgia R. Downstream signalling and specific inhibition of c-MET/HGF pathway in small cell lung cancer: implications for tumour invasion. Br J Cancer. 2007;97(3):368–77. doi: 10.1038/sj.bjc.6603884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Sekiya T. Accumulation of genetic alterations and their significance in each primary human cancer and cell line. Mutat Res. 1998;400:421–437. doi: 10.1016/s0027-5107(98)00031-1. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Matsubara D, Goto A, Ota S, Sachiko O, Ishikawa S, et al. Constitutive activation of c-Met is correlated with c-Met overexpression and dependent on cell-matrix adhesion in lung adenocarcinoma cell lines. Cancer Sci. 2008;99:14–22. doi: 10.1111/j.1349-7006.2007.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian CN, Guo X, Cao B, Kort EJ, Lee CC, Chen J, et al. Met protein expression level correlates with survival in patients with late-stage nasopharyngeal carcinoma. Cancer Res. 2002;62:589–596. [PubMed] [Google Scholar]
- Stabile LP, Lyker JS, Huang L, Siegfried JM. Inhibition of human non-small cell lung tumors by a c-Met antisense/U6 expression plasmid strategy. Gene Ther. 2004;11:325–335. doi: 10.1038/sj.gt.3302169. [DOI] [PubMed] [Google Scholar]
- Tulasne D, Foveau B. The shadow of death on the MET tyrosine kinase receptor. Cell Death Differ. 2008;15:427–434. doi: 10.1038/sj.cdd.4402229. [DOI] [PubMed] [Google Scholar]
- Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol. 2001;153:1023–1034. doi: 10.1083/jcb.153.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JH, Lui VW, Umezawa K, Ho Y, Wong EY, Ng MH, et al. A small molecule inhibitor of NF-kappaB, dehydroxymethylepoxyquinomicin (DHMEQ), suppresses growth and invasion of nasopharyngeal carcinoma (NPC) cells. Cancer Lett. 2009 doi: 10.1016/j.canlet.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:421–429. doi: 10.1016/s1044579x02000858. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Thomas SM, Lui VW, Xi S, Siegfried JM, Fan H, et al. Phosphorylation of TNF-alpha converting enzyme by gastrin-releasing peptide induces amphiregulin release and EGF receptor activation. Proc Natl Acad Sci U S A. 2006;103:6901–6906. doi: 10.1073/pnas.0509719103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.