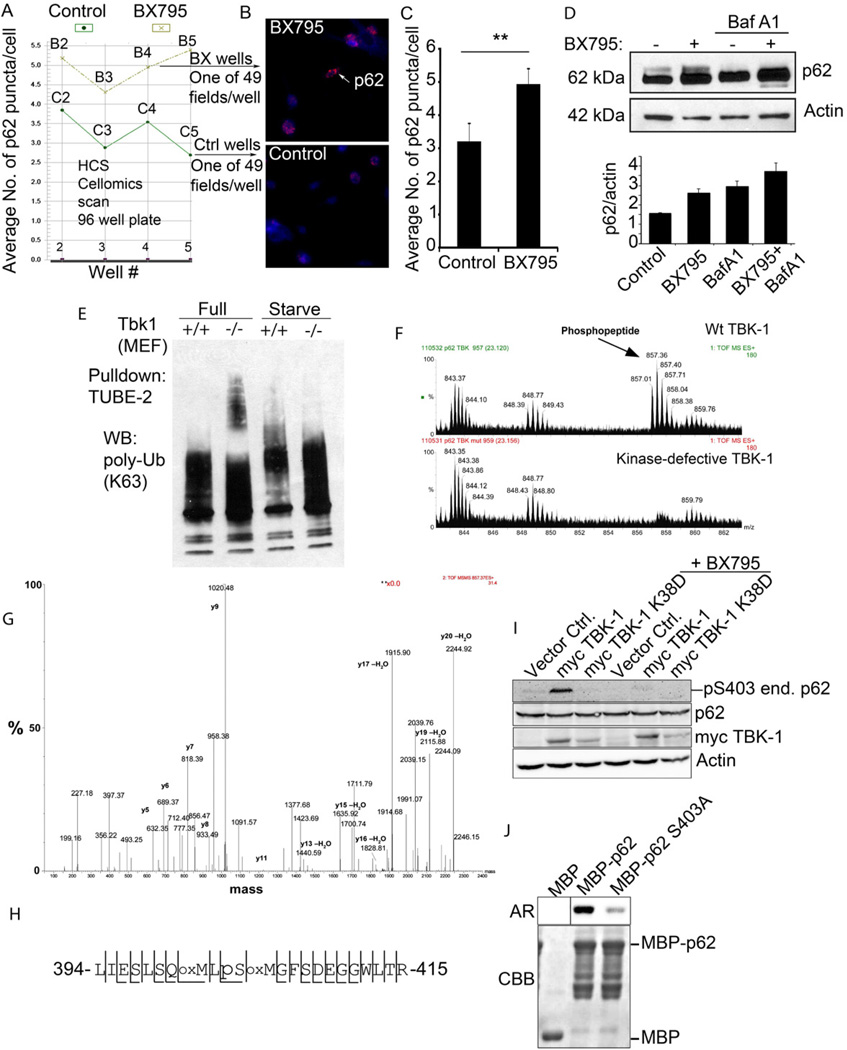
Fig. 5. TBK-1 controls p62 phosphorylation, and affects autophagic clearance of p62 and its cargo capture, delivery and degradation.
A–C. High content imaging analysis (using Cellomics high-content microscopy system) of p62 puncta (endogenous, revealed by imunofluorescence) in BMM with or without treatment with TBK-1 inhibitor BX795. Panel A shows output from Cellomics high-content microscopy and analysis software comparing the number of p62 puncta between TBK-1 inhibitor-treated (BX795) and control (DMSO) BMM. Vertical axis denotes the mean number of p62 puncta per cell and horizontal axis denotes the position of the well (B, BX795 series; C, control series) on the plate. Between 754 and 2395 cells were analyzed per well. Panel C shows t test (data, means ± se; **, p<0.01) from cumulative data treating only whole wells as independent samples (n=4). D. Analysis of the effects of TBK-1 pharmacological inhibitor, BX795 on p62 levels. Tbk-1+/+ MEFs were treated with BX795; bafilomycin A1 (BafA1) to inhibit autophagic degradation. Densitometric analyses of p62 levels normalized against actin levels were plotted (n=2; error bars, range). E. Analysis of TBK-1 role in autophagic clearance of polyubiquitinated proteins. Cell lysates from Tbk1+/+ and Tbk1−/− MEFs uninduced and induced for autophagy by starvation were incubated with TUBE2 agarose beads and bound material pulled down. Protein blots were probed for K63 polyubiquitin chains. F–H. Identification of TBK-1-dependent S303 phosphorylation of the UBA domain of p62. In vivo phosphorylation of p62 UBA domain following cotransfection of GFP-p62D69A (D69A mutation prevents oligomerization with endogenous p62) and expression constructs of TBK-1 wild type or kinase defective form. Immunoprecipitated (GFP-p62) material was subjected to tandem mass spectrometry. A triply charged ion with the mass 857.01 was selected for fragmentation. This ion was identified as the phosphorylated LIESLSQMLpSMGFSDEGGWLTR peptide (shown in panel H) from p62. Panel G shows MS spectra from LC-MS, showing the phosphopeptide of 857.01 m/z observed in p62 phosphorylated by TBK-1. The peptide was not observed when GFP-p62 was co-transfected with the kinase-defective K38D mutant of TBK-1. Spectra are taken from the same retention time in both runs, confirmed by the unspecific peaks observed in both spectra. I. HEK293 cells transfected with vector control, myc-TBK-1 or myc-TBK-1 K38D were left untreated or were treated for 2 h with 1 µM BX795. Cell extracts were immunoblotted with antibodies against phospho-p62 (S303), p62, myc and actin. Abbreviations: end. p62, endogenous p62. J. MBP or MBP-tagged p62 proteins were expressed and affinity-purified from E. coli. TBK-1 mediated phosphorylation was assessed by incubating recombinant MBP, MBP-p62 or MBP-p62 S303A with recombinant active TBK-1 in the presence of [γ-32P] ATP for 10 min at 30°C. The reaction products were analyzed by autoradiography (AR). CBB, Coomassie Brilliant Blue staining.