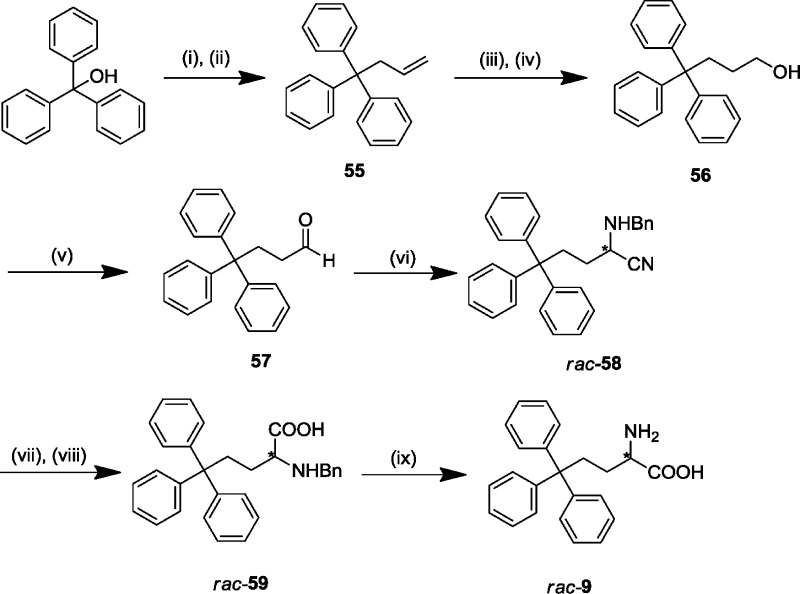
Scheme 3.
Reagents and conditions: (i) n-BuLi, CH2Cl2, rt, 30 min; (ii) allyltrimethylsilane, FeCl3, rt, 6 h; (iii) NaBH4, conc H2SO4 in Et2O, diglyme, rt, 3.5 h, then 75 °C, 1.5 h; (iv) 30% aq H2O2, 3 M aq NaOH, rt, 6.5 h; (v) Dess–Martin periodinane, CH2Cl2, rt, 3 h; (vi) benzylamine, TMSCN, Montmorillonite KSF clay, CH2Cl2, rt, 2 h; (vii) 6 M HCl in dioxane, reflux, 48 h; (viii) propylene oxide, EtOH, reflux, 30 min; (ix) HCOONH4, 10% Pd/C, MeOH, 80 °C, 1 h.