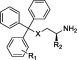
Table 2. STLC Analogues with Modified Trityl Ringsa.
| compd | X | R1 | R2 | inhibition of basal ATPase activity, Kiapp (nM) | ligand efficiency | K562 cells, GI50 (nM) |
|---|---|---|---|---|---|---|
| 11 | S | 2-Cl | (R)-CO2H | 1783.9 ± 384.0 | 0.29 | nd |
| 12 | S | 3-F | H | 377.4 ± 38.4 | 0.36 | nd |
| 13 | S | 3-Cl | (R)-CO2H | 89.9 ± 18.5 | 0.36 | 2404 ± 222 |
| 14 | S | 3-Cl | H | 297.8 ± 60.0 | 0.37 | 1045 ± 42 |
| 15 | S | 3-Br | H | 293.9 ± 61.8 | 0.37 | nd |
| 16 | S | 3-Me | H | 80.0 ± 23.9 | 0.40 | 698 ± 115 |
| 17 | S | 3-Et | H | 5.9 ± 2.3 | 0.45 | 680 ± 84 |
| 18 | S | 3-i-Pr | H | 10.3 ± 3.4 | 0.42 | 581 ± 68 |
| 19 | S | 3-n-Pr | H | 29.6 ± 11.0 | 0.39 | 1760 ± 124 |
| 20 | S | 3-CF3 | H | 352.7 ± 27.9 | 0.33 | nd |
| 21 | S | 3-OMe | H | 149.8 ± 18.5 | 0.37 | nd |
| 22 | S | 3-SMe | H | 520.4 ± 87.6 | 0.34 | 1474 ± 330 |
| 23 | S | 3-OCF3 | H | 27.8 ± 5.0 | 0.37 | 1518 ± 164 |
| 24 | S | 3-COMe | (R)-CO2H | 519.8 ± 102.0 | 0.30 | 964 ± 82 |
| 25 | S | 3-COMe | H | 185.8 ± 30.4 | 0.35 | 706 ± 47 |
| 26 | S | 4-COMe | (R)-CO2H | 271.7 ± 36.6 | 0.31 | 4266 ± 274 |
| 27 | S | 4-COMe | H | 51.0 ± 21.6 | 0.38 | 705 ± 77 |
| 28 | C | 3-Cl | H | 120.6 ± 20.7 | 0.35 | 596 ± 68 |
| rac-29 | C | 3-Me | CO2H | 12.2 ± 3.8 | 0.40 | 73 ± 3 |
| (S)-29 | C | 3-Me | (S)-CO2H | 11.1 ± 3.9 | 0.40 | 128 ± 15 |
| (R)-29 | C | 3-Me | (R)-CO2H | 6.4 ± 3.9 | 0.41 | 91 ± 9 |
| 30 | C | 3-Me | H | 8.8 ± 1.8 | 0.46 | 200 ± 16 |
| 31 | C | 4-Cl | H | 16.2 ± 3.1 | 0.44 | 337 ± 28 |
| rac-32 | C | 4-Me | CO2H | 7.5 ± 1.7 | 0.41 | 96 ± 5 |
| (S)-32 | C | 4-Me | (S)-CO2H | 16.7 ± 3.0 | 0.39 | 149 ± 6 |
| (R)-32 | C | 4-Me | (R)-CO2H | 5.4 ± 1.7 | 0.42 | 82 ± 4 |
| 33 | C | 4-Me | H | 16.4 ± 1.9 | 0.44 | 219 ± 21 |
nd = not determined.