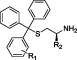
Table 3. STLC Analogues with Modified Trityl Ringsa.
| compd | R1 | R2 | inhibition of basal ATPase activity, Kiapp (nM) | ligand efficiency | K562 cells, GI50 (nM) |
|---|---|---|---|---|---|
| 34 | 2-OH | CO2H | 1978.5 ± 587.3 | 0.29 | nd |
| 35 | 3-OH | CO2H | 48.8 ± 22.0 | 0.37 | 2559 ± 302 |
| 36 | 3-OH | H | 200.3 ± 51.9 | 0.38 | 555 ± 121 |
| 37 | 3-CN | H | 450.6 ± 197.4 | 0.35 | 2128 ± 108 |
| 38 | 3-CH2NH2 | H | 838.2 ± 164.1 | 0.33 | 2018 ± 244 |
| 39 | 3-CH2NHCOMe | H | 829.2 ± 100.5 | 0.30 | 2133 ± 135 |
| 40 | 3-CO2H | H | 990.6 ± 156.9 | 0.31 | 2138 ± 241 |
| 41 | 3-CONH2 | CO2H | 329.9 ± 49.2 | 0.30 | 16749 ± 6112 |
| 42 | 3-CONH2 | H | 419.7 ± 38.8 | 0.33 | 802 ± 51 |
| 43 | 3-CONHMe | H | 887.8 ± 74.9 | 0.31 | 982 ± 72 |
| 44 | 3-CONMe2 | H | 6055.6 ± 1123.0 | 0.25 | 2831 ± 171 |
| 45 | 3-SO2Me | H | 2089.6 ± 246.6 | 0.29 | 2559 ± 180 |
| 46 | 4-CN | H | 432.4 ± 91.2 | 0.35 | 2178 ± 149 |
| 47 | 4-CH2OH | H | 311.2 ± 31.8 | 0.35 | 783 ± 50 |
| 48 | 4-CH2NH2 | H | 2942.4 ± 782.8 | 0.30 | 2594 ± 191 |
| 49 | 4-CH2NHCOMe | H | 1721.3 ± 294.5 | 0.28 | 2904 ± 150 |
| 50 | 4-CONH2 | H | 3228.6 ± 447.4 | 0.29 | 4335 ± 341 |
| 51 | 4-CONHMe | H | 2030.8 ± 671.6 | 0.29 | 3954 ± 293 |
| 52 | 4-CONMe2 | H | 1526.7 ± 359.7 | 0.28 | 2911 ± 271 |
| 53 | 4-SO2Me | H | 1212.1 ± 179.9 | 0.30 | 2735 ± 482 |
nd = not determined.