| No.a | Structureb | TR IC50 (μM)c |
GR IC50 (μM)c |
Selectivity for TRe |
T. brucei EC50 (μM)f |
||
|---|---|---|---|---|---|---|---|
|
| |||||||
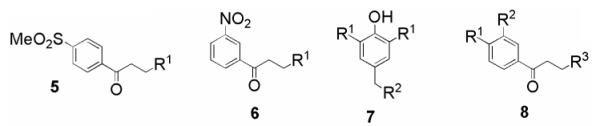
| R1 | R2 | R3 | |||||
| 5a | NMe2.HCl | - | - | 2.9 (±1.4) | 20.4 (±6.3) | 7 | 0.55 (±0.04) |
| 5b | N-piperidyl | - | - | 7.8 (±3.7) | 105 (±36) | 13 | 0.95 (±0.09) |
| 6a | NMe2.HCl | - | - | 0.34 (±0.13) | 20.3 (±8.4) | 59 | 0.68 (±0.10) |
| 6b | NH-4-(CO2-nBu)Ph | - | - | 2.4 (±0.92) | > 135d | >56 | 0.96 (±0.11) |
| 6c | NHPh | - | - | 1.7 (±0.78) | 58.0 (±16) | 35 | 1.01 (±0.07) |
| 6d | NH-2,3-diMePh | - | - | 1.5 (±0.71) | 86.3 (±41) | 58 | 5.87, 0.68, 0.15g |
| 6e | NH-3-FPh | - | - | 3.3 (±1.1) | > 173 | >52 | 0.82 (±0.16) |
| 7a | tBu | N((CH2)2OH).HCl | - | 2.0 (±0.21) | 24.9 (±6.0) | 13 | 3.01 (±0.30) |
| 7b | iPr | NMe2 | - | 11.8 (±3.2) | 79.3 (±25) | 6.7 | > 50 |
| 7c | tBu | N-homopiperidyl | - | 38.8 (±5.0) | > 155 | >4 | 3.30 (±0.30) |
| 8a | Cl | Cl | R3 = NMe2.HCl | 3.3 (±2.2) | 64.8 (±18) | 20 | 1.00 (±0.21) |
| 8b | Cl | H | R3 = N-morpholinyl | 148 (±31) | > 212 | >1.4 | 0.98 (±0.07) |
| 8c | Cl | Cl | R3 = NH-4-EtPh | 107 (±0.71) | > 157 | >1.5 | 1.65 (±0.18) |
All compounds were purchased from ChemBridge, except for 5a and 5b (Peakdale).
Dash signifies that this chemotype did not possess variable groups at this position.
Values are means of two experiments, standard deviation is given in parentheses.
IC50 value greater than the highest tested inhibitor concentration.
GR IC50/TR IC50.
Values are weighted means of three independent experiments, standard errors are given in parentheses. IC50 values for pentamidine are 4.6 (± 0.2) nM.
Compound 6d gave highly variable EC50 results, all three are listed.
