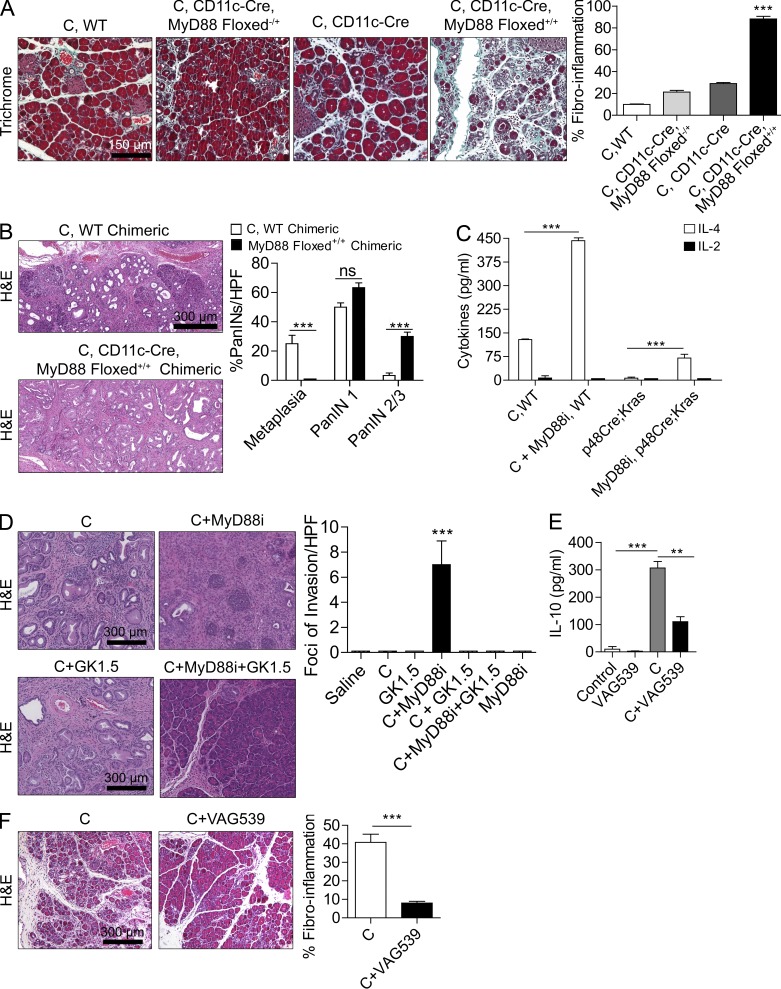
Figure 4.
MyD88 blockade within DCs exacerbates pancreatic disease in a CD4+ T cell–dependent manner. (A) 4-wk-old CD11c-Cre MyD88 Floxed+/+ mice and control animals were treated with caerulein (C) for 3 wk to induce chronic pancreatitis. Pancreata were examined by Trichrome staining, and the fibroinflammatory area was quantified (n = 4 mice/group; ***, P < 0.001). (B) p48Cre;KrasG12D mice were made chimeric using bone marrow derived from WT or CD11c-Cre MyD88 Floxed+/+ mice. Chimeric mice were treated with caerulein and sacrificed at 3 wk (n = 4–5/group). Representative images are shown, and the fraction of graded PanIN lesions was quantified (***, P < 0.001). (C) CD4+ T cells were harvested from the pancreata of p48Cre;KrasG12D mice and caerulein-treated WT mice that were administered MyD88 inhibitory peptide or control peptide. IL-2 and IL-4 levels were measured in cell culture supernatant (***, P < 0.001). (D) 4-wk-old p48Cre;KrasG12D mice were treated with C, C + MyD88 inhibitor, C + GK1.5 to deplete CD4 T cells, C + MyD88 inhibitor + GK1.5, or additional controls. Mice were sacrificed at 3 wk, and the foci of invasive cancer were quantified by examining 10 HPFs per mouse (n = 5 mice/group; ***, P < 0.001). (E) IL-10 was measured in cell culture supernatant from purified pancreatic CD4+ T cells from mice treated for 2 d with saline, VAG539, caerulein, or caerulein + VAG539 (***, P < 0.001). (F) WT mice were treated with caerulein alone or caerulein + VAG539 for 3 wk. Representative H&E-stained sections are shown (n = 4 mice/group), and fibroinflammatory changes were quantified (***, P < 0.001). Error bars indicate standard error of the mean.