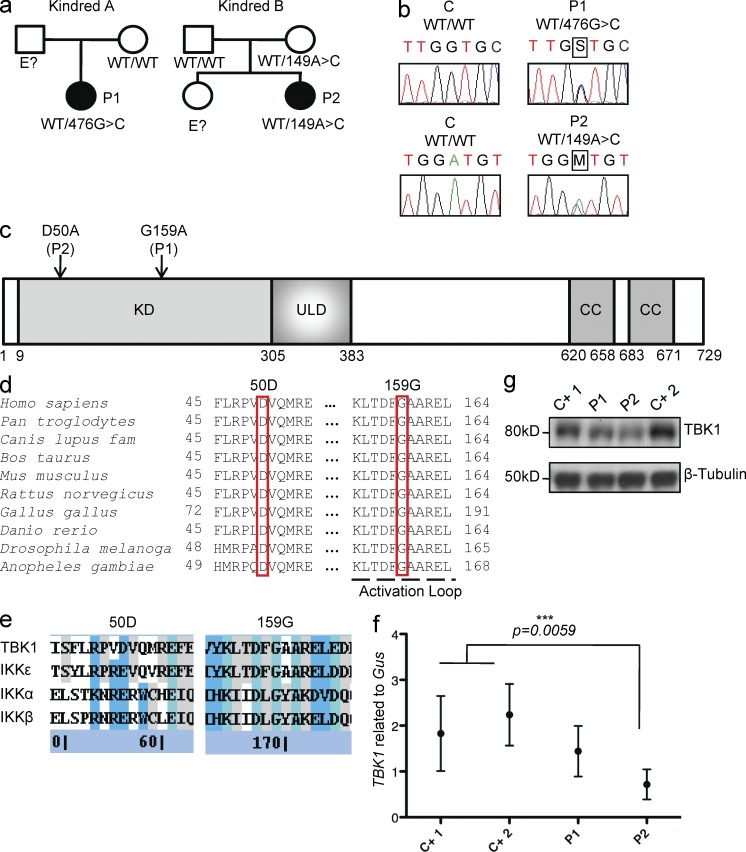
Figure 1.
Heterozygous TBK1 mutations in two children with HSE. (a) Family pedigrees and segregation. (b) Heterozygous TBK1 mutations 476G>C in P1 and 149A>C in P2. The PCR products sequenced were amplified from genomic DNA from the granulocytes of a control (C) and both patients. (c) Schematic diagram of the protein structure of TBK1, featuring its kinase domain (KD), ubiquitin-like domain (ULD), and coiled-coil (CC) regions. Both heterozygous substitutions, 159G>G/A (P1) and 50D>D/A (P2), affect the kinase domain of TBK1 (amino acids 9–305). (d) Multiple alignments of relevant amino acid sequences of the kinase domain of human TBK1 with its homologues from nine other species, with the residues mutated in P1 (G159) and P2 (D50) highlighted. (e) Multiple alignments of relevant amino acid sequences of the kinase domain of human TBK1 with the other IKK and IKK-related kinases, IKK-α (46% similar to TBK1), IKK-β (44% similar), and IKK-ε (64% similar). The residues mutated in P1 and P2 are conserved (G159) or similar (D50) across IKK or IKK-related kinases. (Blue signifies sequence similarity, teal signifies sequence identity, and gray signifies partial identity/similarity.) (f) TBK1 expression, as assessed by RT-qPCR on mRNA from the SV40-fibroblasts of patients (P1 and P2) and control lines (C+1 and C+2). Values represent mean values ± SD calculated from three independent experiments. (g) TBK1 levels, as assessed by Western blotting, in SV40-fibroblasts from patients (P1 and P2) and two control lines (C+ 1 and C+ 2). This Western blot result is representative of three experiments.