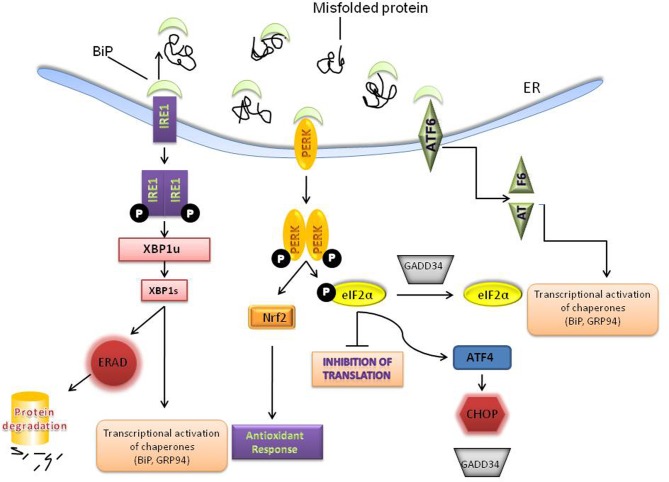
Figure 1.
Activation of the unfolded protein response (UPR). Accumulation of misfolded or unfolded proteins in the ER leads to the dissociation of BiP from 3 transducers –PERK, IRE1 and ATF6. PERK homodimerizes and phosphorylates eIF2α to inhibit general protein translation. PERK also regulates several transcription factors including, NRF-2 to up-regulate the anti-oxidant response and ATF4 which can lead to both protective and apoptotic signaling. IRE-1 activation results in the unconventional splicing of XBP-1, which induces the transcription of several molecular chaperones, such as BiP and GRP94 and stimulates protein degradation via ER-associated degradation (ERAD). ATF6 is activated and cleaved and leads to induction of molecular chaperones. The various ER chaperones are part of a protective adaptive response that regulates protein folding and other components of the UPR.