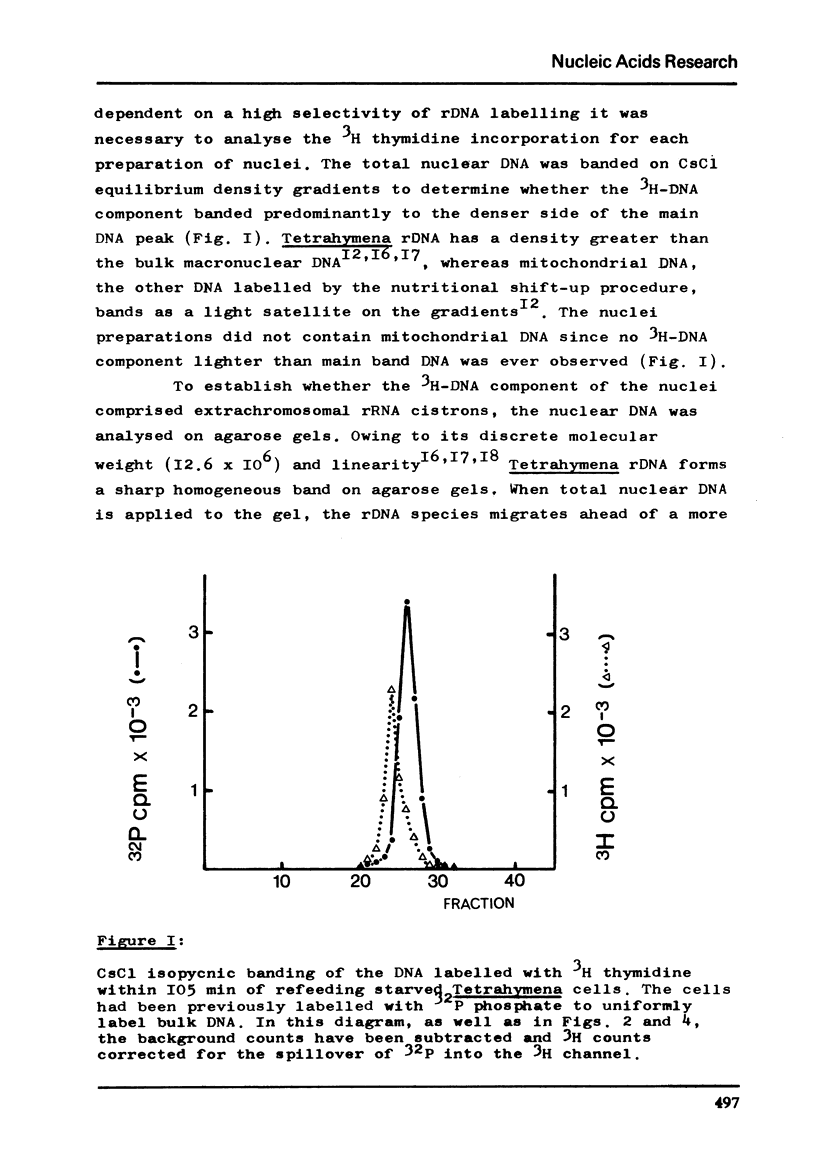
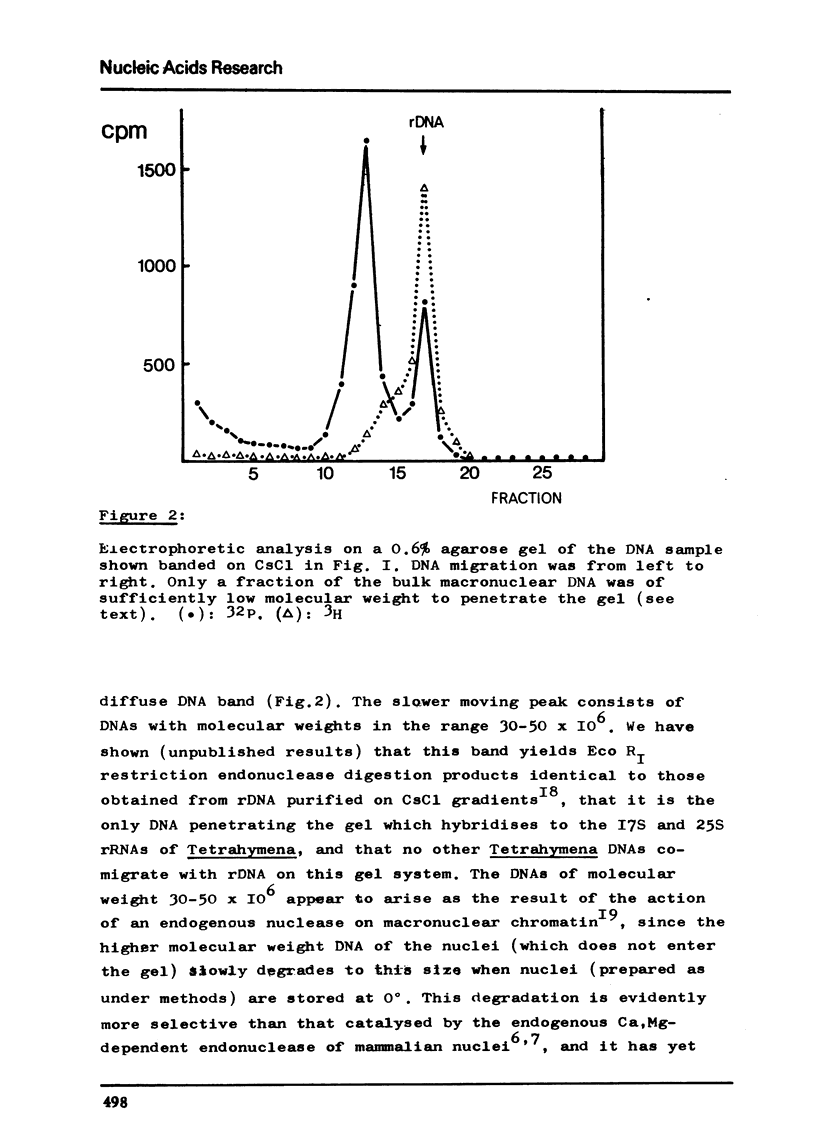
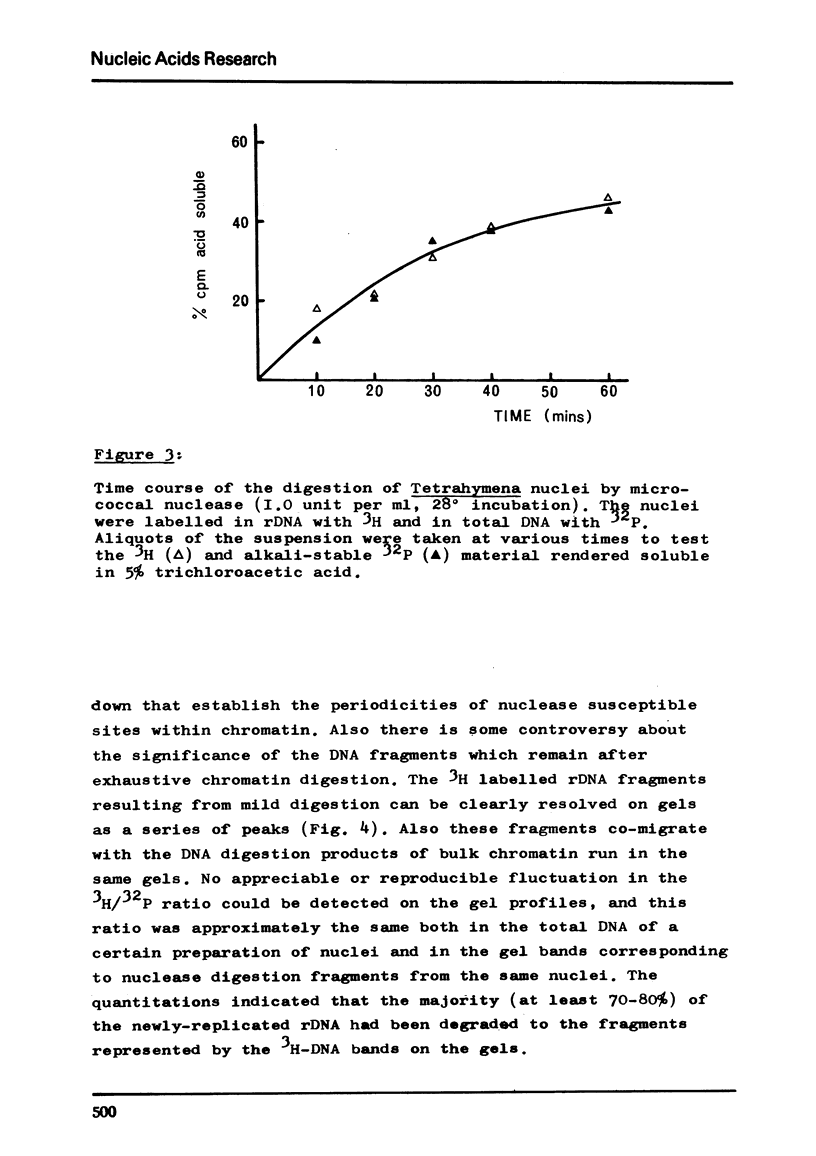
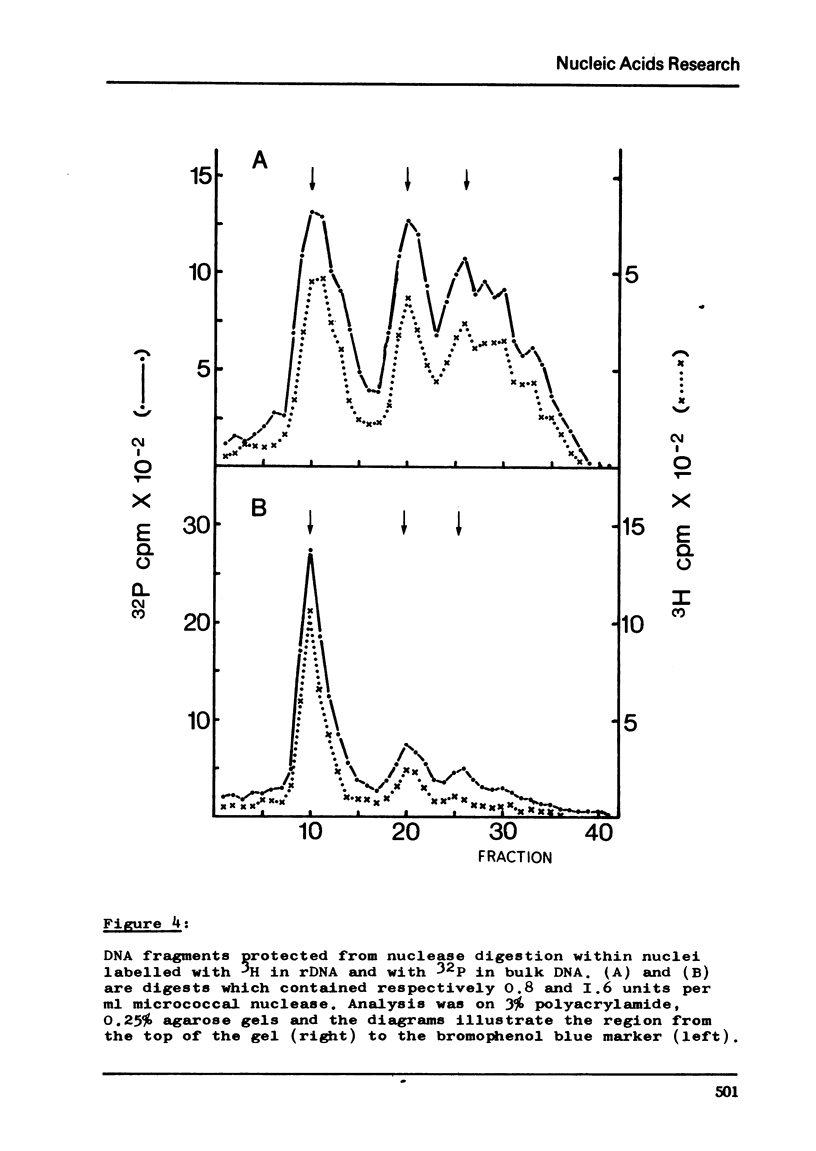
Abstract
Synchronised cells of Tetrahymena pyriformis GL were labelled with 3H thymidine at a stage in the cell cycle when only the mitochondrial and extrachromosomal nucleolar ribosomal DNAs were replicating. In this way it was possible to prepare nuclei labelled selectively in the DNA of the ribosomal RNA genes. Since the ribosomal RNA cistrons of these cells are also very active in serving as a template for transcription, experiments were performed to test whether these genes are organised upon a nucleoprotein subunit structure of the kind that has been found in the total chromatin of a wide range of eukaryotic cell types. Tetrahymena macronuclei were prepared labelled uniformly in their DNA with 32P and labelled only in their nucleolar ribosomal DNA with 3H. Both the ribosomal genes and the bulk chromatin were then degraded in situ using micrococcal nuclease. The DNA fragments resulting from mild digestion were analysed on gels to reveal an identical DNA degradation pattern within both the ribosomal and bulk chromatins. It is concluded that the nucleoprotein structure of nucleolar rRNA cistrons posesses a periodic repeat along the DNA which is identical to that found in the substructure of unfractionated chromatin.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen H. A., Engberg J. Timing of the ribosomal gene replication in Tetrahymena pyriformis. Exp Cell Res. 1975 Apr;92(1):159–163. doi: 10.1016/0014-4827(75)90649-7. [DOI] [PubMed] [Google Scholar]
- Axel R., Melchior W., Jr, Sollner-Webb B., Felsenfeld G. Specific sites of interaction between histones and DNA in chromatin. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4101–4105. doi: 10.1073/pnas.71.10.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg J., Christiansen G., Leick V. Autonomous rDNA molecules containing single copies of the ribosomal RNA genes in the macronucleus of Tetrahymena pyriformis. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1356–1365. doi: 10.1016/0006-291x(74)90463-x. [DOI] [PubMed] [Google Scholar]
- Engberg J., Nilsson J. R., Pearlman R. E., Leick V. Induction of nucleolar and mitochondrial DNA replication in Tetrahymena pyriformis. Proc Natl Acad Sci U S A. 1974 Mar;71(3):894–898. doi: 10.1073/pnas.71.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. T., Noll M., Kornberg R. D. Electron microscopy of defined lengths of chromatin. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3320–3322. doi: 10.1073/pnas.72.9.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G. Free ribosomal RNA genes in the macronucleus of Tetrahymena. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3078–3081. doi: 10.1073/pnas.71.8.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Keevert J. B. Subunit structure of a naturally occurring chromatin lacking histones F1 and F3. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3536–3540. doi: 10.1073/pnas.72.9.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M., Garrard W. T., Bagi G., Wilson R. F., Bonner J. Partial purification of the template-active fraction of chromatin: a preliminary report. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2193–2197. doi: 10.1073/pnas.71.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M., Murphy R. F., Bonner J. Structure of transcriptionally active chromatin. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4404–4408. doi: 10.1073/pnas.72.11.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D. Chromatin structure: deduced from a minichromosome. Science. 1975 Mar 28;187(4182):1202–1203. doi: 10.1126/science.187.4182.1202. [DOI] [PubMed] [Google Scholar]
- Hamkalo B. A., Miller O. L., Jr Electronmicroscopy of genetic activity. Annu Rev Biochem. 1973;42:379–396. doi: 10.1146/annurev.bi.42.070173.002115. [DOI] [PubMed] [Google Scholar]
- Hayward G. S. Gel electrophoretic separation of the complementary strands of bacteriophage DNA. Virology. 1972 Jul;49(1):342–344. doi: 10.1016/s0042-6822(72)80042-4. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Chromatin structure: a repeating unit of histones and DNA. Science. 1974 May 24;184(4139):868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- Lacy E., Axel R. Analysis of DNA of isolated chromatin subunits. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3978–3982. doi: 10.1073/pnas.72.10.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll M. Internal structure of the chromatin subunit. Nucleic Acids Res. 1974 Nov;1(11):1573–1578. doi: 10.1093/nar/1.11.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll M. Subunit structure of chromatin. Nature. 1974 Sep 20;251(5472):249–251. doi: 10.1038/251249a0. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Carlson R. D., Olins D. E. Visualization of chromatin substructure: upsilon bodies. J Cell Biol. 1975 Mar;64(3):528–537. doi: 10.1083/jcb.64.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Kimmel A. R., Gorovsky M. A. A small number of cistrons for ribosomal RNA in the germinal nucleus of a eukaryote, Tetrahymena pyriformis. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3082–3086. doi: 10.1073/pnas.71.8.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]



