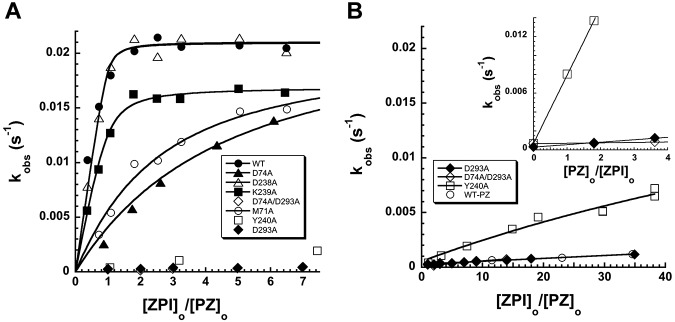
Figure 4.
Kinetic titrations of mutant ZPI binding to PZ. (A) Titrations of the PZ-dependent increase in kobs for wild-type and mutant ZPI reactions with factor Xa (0.04nM) in the presence of 8.8nM PZ, variable ZPI, 25μM phospholipid, and 1mM Ca2+. (B) Titrations of the low-PZ-affinity variants of ZPI from panel A over an extended range of ZPI concentrations, along with control wild-type ZPI in the absence of PZ. Inset shows titrations of the PZ-dependent increase in kobs for reactions of low-PZ-affinity ZPI variants with factor Xa at 55nM ZPI, variable PZ, 25μM phospholipid, and 1mM Ca2+. kobs values represent averages of 2-3 independent measurements. Solid lines are fits of data by the equation given in “Kinetics of ZPI-FXa reactions” from which values of KD for the ZPI-PZ interaction and kass,cat were determined (Table 1). For ZPI variants with low PZ affinity, kass,cat was fixed at the wild-type value.