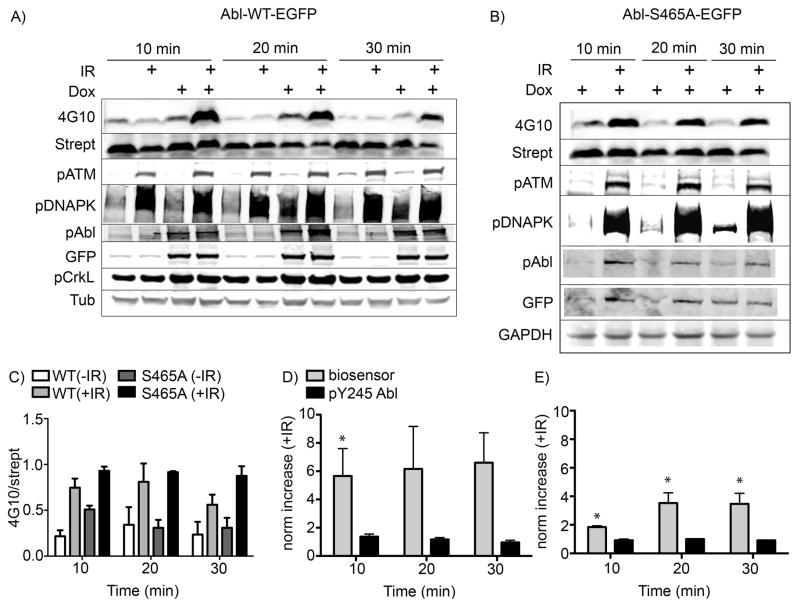
Figure 2. Phosphorylation of the Abl kinase biosensor after exposure to IR.
The Abl-WT-EGFP and Abl-S465A-EGFP cells were prepared as described in the experimental section. The peptide substrate (25μM) was added and cells were incubated at 37°C for 5 min, then treated with IR (5 Gy). Cells were harvested at different times post IR treatment as indicated. A) Western blot analysis using quantitative two-color Licor IR-dye imaging showed that the phosphorylation of the biosensor peptide (detected via 4G10 signal just below the 6 kDa molecular weight marker, confirmed via overlaid signal from streptavidin visualized using the second channel of the Licor Odyssey scanner) was dramatically increased after IR treatment in cells overexpressing the Abl-WT-EGFP construct (row 1, lanes 4, 8 and 12). B) Unexpectedly, biosensor phosphorylation was still increased in cells expressing the Abl-S465A-EGFP mutant protein (row 1, lanes 2, 4 and 6). Immunoblotting for other proteins served as controls for IR-induced pathway and Abl activation (pATM, pDNAPK, pCrkL), construct expression/autophosphorylation (GFP, Abl(pY245)) and total protein loading (Tubulin or GAPDH). Total ATM and DNAPK blots showed consistent levels of the unphosphorylated forms of these proteins (data not shown). C), D) and E) Integrated band intensity data for the 4G10, streptavidin, phospho-c-Abl(Y245) and anti-GFP signals over independent replicate experiments were calculated and the phosphorylated signals (4G10 and phospho-c-Abl(Y245)) were divided by their respective ‘total’ marker signals (streptavidin, labelled as strept, and anti-GFP, respectively) and plotted together. Axes are labelled as 4G10/strept or norm increase (+IR), meaning the increase in the 4G10/strept signal for samples treated with IR vs non-IR controls. Error bars represent SEM, and statistical significance (p<0.05) of the change vs. non-irradiated cells is indicated using *. For the Abl-WT-EGFP biosensor data, N = 5 and for the phospho-Y245 data N = 3. For the Abl-S465A-EGFP biosensor data, N = 4 and for the phospho-Y245 data, N = 2.