Table 2.
Substrate Scope of the Intramolecular Amination of Allylic Alcohols Catalyzed by a 1:1 Mixture of (1)AuCl and AgSbF6 (5 mol %) in Dioxane.
| entry | substrate | temp (°C) | time (h) | heterocycle | yield (%)a | drb |
|---|---|---|---|---|---|---|
| 1 |
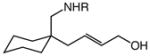 4a (R = Bn) |
23 | 2 |
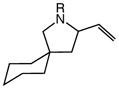 5a |
94 | – |
| 2 | 4b [R = CH(Me)Ph] | 23 | 2 | 5b | 99 | 2:1 |
| 3 |
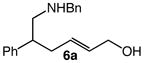 6a |
60 | 8 |
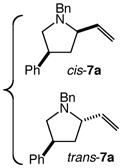
|
54 | – |
| 41 | – | |||||
| 4 |
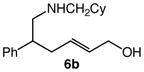 6b |
60 | 8 |
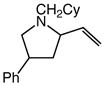 7b |
99 | 1.5:1 |
| 5 |
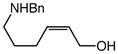 8 (Z/E = 7.7:1) |
60 | 12 |
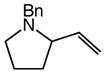 9 |
86 | – |
| 6 |
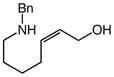 10 |
60 | 16 |
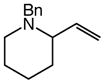 11 |
91 | – |
| 7 |
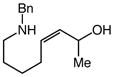 12 |
100 | 14 |
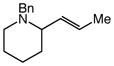 13 |
99 | ≥50:1 |
| 8 |
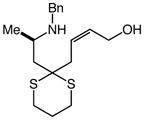 14 |
100 | 48 |
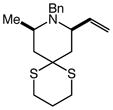 15 |
91 | 25:1 |
Isolated yield in >95% purity.
Diastereomeric ratio determined by 1H NMR analysis of the crude reaction mixture.
