Table 2.
Substrate Scope of the Intramolecular Amination of Allylic Alcohols (0.6 M) Catalyzed by a 1:2 Mixture of [(S)-2](AuCl)2 (2.5 mol %) and AgClO4 (5 mol %) in Dioxane at 25 °C for 48 h.[18]
| entry | substrate | heterocycle | yield [%][a] | ee [%][b] |
|---|---|---|---|---|
| 1 |
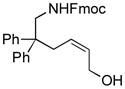 (Z)-1g |
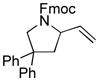 3g |
95 | Š5 |
|
|
|||
| 2 | (R = Me) | 94 | 90 | |
| 3 | [R-R = –(CH2)5–] | 95 | 94 | |
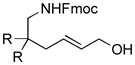
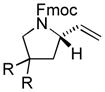
| 4 |
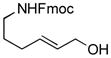
|
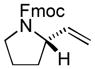
|
89 | 62 |
| 5 |
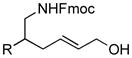 4 (R = Ph) |
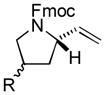 5 |
87[c] | 90/92 |
| 6 | (R = i-Pr) | 98[c] | 85/91 | |
| 7 |
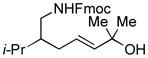
|
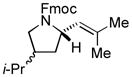
|
87[c] | 88/90 |
| 8 |
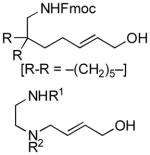
|
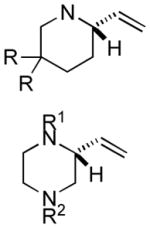
|
69 | 77 |
| 9[d] | (R1 = Fmoc, R2 = Boc) | 86 | 94 | |
| 10[d] | (R1 = Fmoc, R2 = Ts) | 99 | 92 | |
| 11[d] | (R1 = Boc, R2 = Fmoc) | 99 | 91 |
Isolated yield.
Determined by HPLC analysis on chiral support.
Diastereomeric ratio = ~1:1.
Reaction run at 50 °C.
