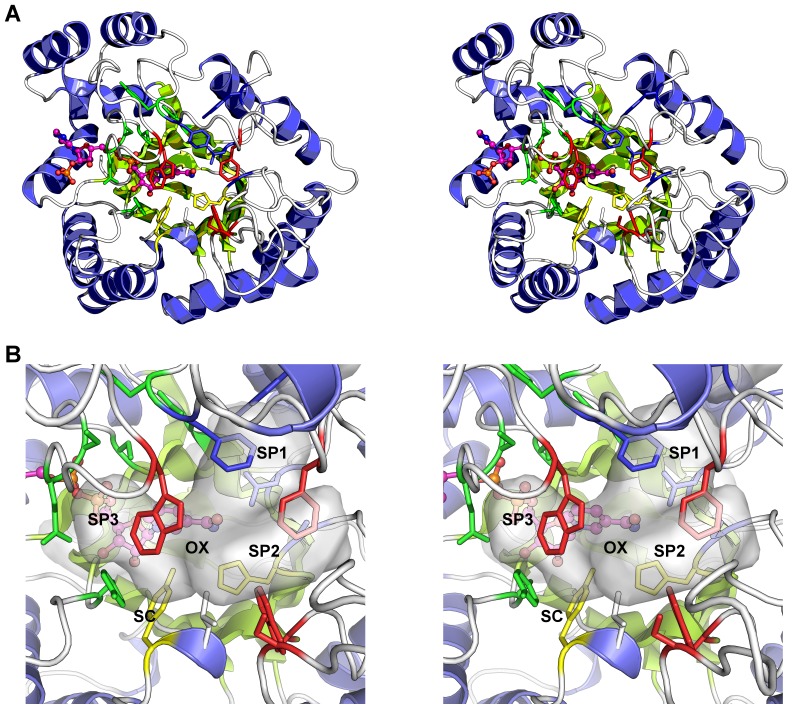
Figure 2. Overall structure and active site of AKR1C3.
A. Cartoon diagram of the protein structure highlighting the active site residues - shown as multicoloured sticks. The NADP+ molecule is shown as a ball and stick figure with carbon atoms coloured magenta. Alpha helices are coloured blue and beta sheets in green. B. Close up view of the active site showing the sub-pocket structure as a semi-transparent surface. The residues lining sub-pocket 1 (SP1) are coloured blue, those lining sub-pocket 2 (SP2) are coloured red, and the sub-pocket 3 (SP3) residues are coloured green. Residues Tyr-55 and His-117 (in yellow sticks), along with the NADP+ molecule, form the oxyanion site (OX), and the steroid channel (SC), the binding site of steroid molecules, is “gated” by residues Trp-227 (foreground, red sticks) and Leu-54 (white sticks). Figures drawn using Pymol v1.3 incentive (Schrödinger, LLC).