Abstract
Purpose
Unilateral peripheral prisms for homonymous hemianopia (HH) place different images on corresponding peripheral retinal points, a rivalrous situation in which local suppression of the prism image could occur and thus limit device functionality. Detection with peripheral prisms has primarily been evaluated using conventional perimetry where binocular rivalry is unlikely to occur. We quantified detection over more visually complex backgrounds and examined the effects of ocular dominance.
Methods
Detection rates of 8 participants with HH or quadranopia and normal binocularity wearing unilateral peripheral prism glasses were determined for static perimetry targets briefly presented in the prism expansion area (in the blind hemifield) and the seeing hemifield, under monocular and binocular viewing, over uniform gray and more complex patterned backgrounds.
Results
Participants with normal binocularity had mixed sensory ocular dominance, demonstrated no difference in detection rates when prisms were fitted on the side of the HH or the opposite side (p>0.2), and had detection rates in the expansion area that were not different for monocular and binocular viewing over both backgrounds (p>0.4). However, two participants with abnormal binocularity and strong ocular dominance demonstrated reduced detection in the expansion area when prisms were fitted in front of the non-dominant eye.
Conclusions
We found little evidence of local suppression of the peripheral prism image for HH patients with normal binocularity. However, in cases of strong ocular dominance, consideration should be given to fitting prisms before the dominant eye. Although these results are promising, further testing in more realistic conditions including image motion is needed.
Keywords: Peli prism, EP prism, low vision, rehabilitation, prism treatment, perimetry, and visual field loss, hemianopsia, quadranopsia, quadrantanopia, traumatic brain injury, TBI, stroke
INTRODUCTION
Homonymous hemianopia (HH) is the congruous loss of half the visual field on one side in both eyes resulting from post-chiasmal lesions. The overall prevalence of HH in individuals over 49 years of age is 0.8%.1 The most common etiologies are stroke (accounting for 70% of cases) followed by traumatic brain injury (13%).2 Patients with HH have difficulty detecting obstacles on the side of the field loss resulting in impaired walking, and are precluded from driving in many U.S states.3 Thus HH can significantly impact a patient’s quality of life.4–6 Spectacle-mounted prisms in a variety of unilateral and bilateral designs, aiming to expand or relocate the visual field, are a commonly applied rehabilitation treatment.7–10 The prisms shift the image of objects located in the blind field into an area of the seeing field.
In 2000, Peli11 described a new approach to fitting prisms for HH – peripheral prism glasses - in which two high-powered prism segments are placed above and below the line of sight on the spectacle lens (Figure 1). When fitted unilaterally the prisms create peripheral diplopia (two views of the same object at different positions in visual space) and visual confusion (the appearance of two different images in the same position). It is the confusion that provides the visual field expansion that can be measured with perimetry. This expansion has been reported to be helpful for obstacle detection when walking,4, 11–13 and may also be helpful for detection of potential hazards when driving.14
Figure 1.

Press-on Fresnel 40Δ prisms, as fitted for the study, placed base left on the left spectacle lens of a left HH patient (the pointed edge of the prism segments indicates the direction of the base).
In visual confusion, images of different objects fall on corresponding retinal points, a situation which may result in binocular rivalry. In the case of peripheral prisms, the prism-shifted image from the prism eye and the normal view of the scene from the non-prism eye fall on peripheral corresponding points which may give rise to two peripheral areas (“islands”) of binocular rivalry. Due to the poor optical quality of high-power Fresnel prisms,15 the prism image is of lower contrast than the image from the non-prism eye, and is therefore less likely to predominate during binocular rivalry.16 Exclusive visibility of a target (dominance/suppression) increases with retinal eccentricity.17 In the extreme case, persistent local suppression of the prism image under binocular viewing conditions would severely reduce the utility of the peripheral prism glasses. However, even a decrease in predominance could potentially reduce device utility somewhat.
To date, detection performance with peripheral prism glasses has primarily been measured using kinetic Goldman perimetry presenting a spot of light on a plain uniform background.4, 11, 12 Under these conditions local suppression of the prism image has not been found, as there is little or no stimulus for rivalry. However, these conditions are not representative of the real world where prism glasses are used to detect objects against visually complex backgrounds. By comparing detection rates for monocular and binocular viewing, we examined whether there was decreased predominance (or persistent local suppression) of the unilateral prism image, as evidenced by reduced detection rates, when targets were presented over complex, patterned backgrounds and over uniform, gray backgrounds (as used in traditional perimetry). We predicted that due to rivalry there would be at least partial local suppression of the prism image on the patterned background, but not the gray background.
Peripheral prisms are usually placed in front of the eye ipsilateral to the blind hemifield (i.e., on the left lens for a patient with left HH), which may not necessarily be the sensory dominant eye. Decreased predominance or binocular suppression is more likely for targets presented to the sensory non-dominant eye.18, 19 Previous studies of sensory ocular dominance reported that the majority of observers with normal binocular vision had no clear dominance (i.e., “mixed” dominance).19, 20 In such cases, sensory dominance would be unlikely to cause local suppression of the prism images. However, for people with strong sensory dominance (e.g., secondary to strabismus, amblyopia or significant/pathological difference in vision), fitting the prism to the sensory non-dominant eye could result in local suppression of the prism images and reduced detection of objects of interest seen only through the prism.
Unilateral peripheral prisms were fitted on the lens ipsilateral to and contralateral to the side of the HH. We predicted that for patients with normal binocular vision and mixed ocular dominance, there would be little difference in detection rates for the two prism fitting conditions (ipsilateral and contralateral). However, for participants with strong ocular dominance, we expected that detection rates would be lower (especially on the patterned background) when the prisms were fitted in front of the sensory non-dominant eye than the dominant eye.
METHODS
This study was conducted in accordance with the tenets of the Declaration of Helsinki. All participants signed a consent form approved by the Schepens IRB.
Participants
Participants had stable (>6 months since diagnosis) HH or quadranopic field loss. In addition, they met the following criteria: a visual acuity of 20/50 or better in each eye, no hemi-spatial neglect (Bells Test21 and Schekenberg Line Bisection Test22), no significant cognitive decline (> 24 on Mini-Mental State Examination23), normal binocular vision (no amblyopia, no strabismus), and no visual field loss affecting the seeing hemifield.
Twenty potential participants were recruited and screened from local clinics, of which 8 met all the criteria and were included in the main study (Table 1); 6 completed all study visits and 2 completed testing with the prism in front of only one eye. Three were current peripheral prism wearers and five were fitted with peripheral prisms for the study. In addition, to evaluate the effect of strong ocular dominance (and/or suppression) two participants with left HH who did not meet the criteria for visual acuity and normal binocularity participated. Their data were not included in the main analyses and are presented separately. One was an existing wearer and the other was fitted with peripheral prisms for the study.
Table 1.
Characteristics of the eight participants in the main study
| All Participants (n = 8) | Current wearer (n = 3) | New wearer (n = 5) | |
|---|---|---|---|
| Male, n (%) | 5 (63%) | 2 (67%) | 3 (60%) |
| Age in years, median (range) | 47.5 (16–70) | 55 (36–62) | 40 (16–70) |
| Snellen Binocular BCVA*, median (range) | 20/20 (20/13–20/25) | 20/20 (20/13–20/20) | 20/20 (20/16–20/25) |
| MMSE^ score, median (range) | 30 (26–30) | 30 (30–30) | 30 (26–30) |
| Time in months since onset of HH, median (range) | 90 (72–168) | 72 (72–132) | 96 (84–168) |
| Number with complete HH, n (%) | 3 (38%) | 2 (67%) | 1 (20%) |
| Number with left sided loss, n (%) | 4 (50%) | 3 (100%) | 1 (20%) |
| Stroke etiology of HH, n (%) | 7 (88%) | 3 (100%) | 4 (80%) |
| Continued wear after study, n (%) | 5 (63%) | 3 (100%) | 2 (40%) |
BCVA = Best corrected visual acuity.
MMSE = Mini-Mental State Examination
In addition to the screening vision measures (visual acuity, refraction, Goldmann perimetry and binocular vision testing), sensory dominance was evaluated using a test battery19, 20 including the Worth 4 dot test, plus lens test, randot polarized suppression test and the Polarized Mirror Test.24
Peripheral Prisms
Peripheral prism segments were fit unilaterally, first to one lens and then the other of participants’ glasses, primarily used for distance vision. The segments were 8mm (vertical) by 22mm with a standard 12mm inter-prism separation12 (Figure 1). The prism base was always placed toward the side of the HH and was therefore in the same direction (right or left) irrespective of which spectacle lens was used. When the prism was on the lens on the side of the HH, current peripheral prism wearers used their own prism glasses for testing (either 40Δ press-on or, in one case, 57Δ permanent prism glasses); a second equivalent pair was provided for testing with prisms on the opposite side. New wearers were provided with study glasses with 40Δ press-on Fresnel prisms and the side of the first prism fitting (the side of HH or the opposite) was counterbalanced. With the exception of one current peripheral prism wearer (who would not permit us to change the press-on prisms on his glasses), all participants using press-on prisms were fitted with new prisms for the study.
Subjects were encouraged to wear the prism glasses as much as possible between visits (except during prolonged near work or when driving).
Detection Test: Backgrounds and Targets
A computerized tangent-screen perimeter25 was used to perform kinetic and static perimetry presenting targets on a large 1.6×1 m rear projection screen at a 1m viewing distance. The dichoptic measurement capability of this system (using liquid crystal goggles25, 26) was not applied in this study. Instead, for monocular viewing conditions, an eye patch was used to occlude the eye not being tested.
Viewing Backgrounds
Two backgrounds were used: a simple, uniform gray background and a more visually complex, patterned background. For the latter, 1/f0.75 noise images26 (Figure 2) were used that had spatial frequency radially-averaged spectra similar to natural scenes,27 but more stationary distributions of luminance and contrast (which may vary considerably in natural scenes causing large local variations in target visibility). The patterned background changed after each target presentation and, on average, the contrast between the background and the target remained uniform despite changes in target position on the screen. Testing on a normally-sighted participant wearing peripheral prism glasses confirmed that the noise images created a detectable rivalry (using the color-marking technique).
Figure 2.
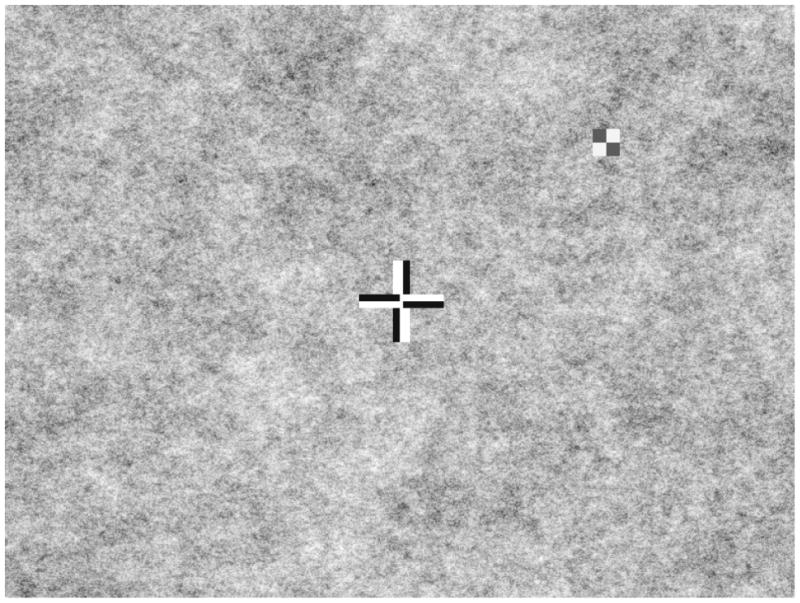
Patient’s view during testing consisting of a patterned background (1/f 0.75 spatial noise), a bipolar fixation cross, and a peripheral bipolar checkerboard target (shown here at high, 95% contrast). Only the central portion of the screen is shown.
Targets
Targets were 1° square checkerboards (2-by-2, black and white 0.5° squares; Figure 2) of 60%, 75%, or 95% internal Michelson contrast. Target contrast was determined separately for each participant before experimental trials began. A contrast level was selected for which detection rates during monocular viewing with peripheral prism glasses were high on the uniform gray background in the seeing hemifield test zone (described below) and were still above 20% on the patterned background in the prism expansion test zone.
Targets were presented for 250 ms, followed by a grace period of 600 ms to allow a subject’s response (a button press). To avoid anticipation of the onset of the next target, a randomized delay of 1000 to 1950 ms occurred after the button press (or grace period if no response) before the next target presentation.
Test Zones
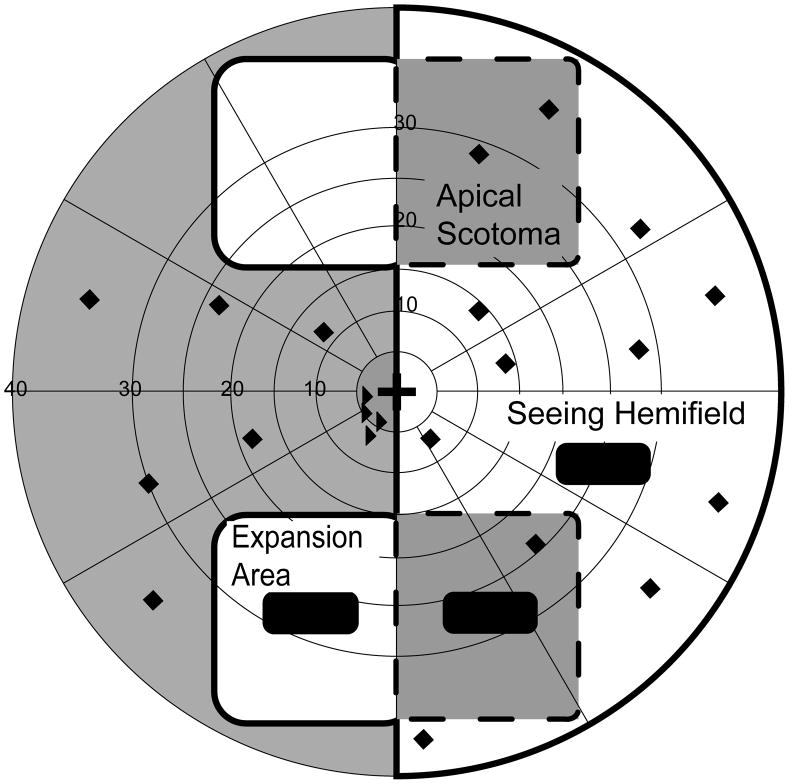
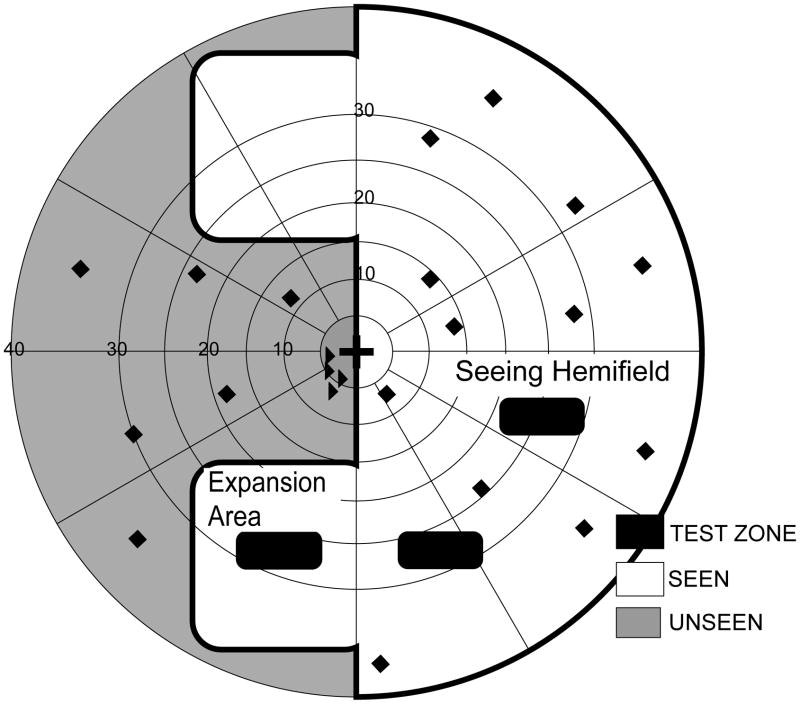
Two main test zones were used: a zone in the blind hemifield to evaluate detection in one prism expansion area (either upper or lower) and a zone within the seeing hemifield in an area where both the prism eye and the fellow eye would be able to detect targets in monocular and binocular conditions (Figure 3). In addition, a third test zone in the seeing hemifield, but under the optical apical scotoma caused by the unilateral prism,28 was used (Figure 3a). In monocular, prism-eye-only viewing we expected that no targets would be detected in this area (thus acting as a fixation check); however, in binocular viewing we expected that the fellow eye would compensate for the apical scotoma.
Figure 3.
Schematic visual field plots and static detection test zones for a patient with left HH wearing peripheral prism glasses. (A) Prism eye only; (B) Binocular viewing. Thick black lines mark the kinetic isopter. Dashed lines outline the prism apical scotoma mapped in the prism-eye only condition. Black-filled rounded-corner rectangles represent test zones (20 targets per zone). Black diamonds represent arbitrary positions of additional targets (20) included to prevent anticipation of a target’s location. Black triangles are targets used to monitor fixation.
All test zones were 3° (vertically) by 8°, deliberately smaller than the expansion areas (of about 24° by 22°) in order to ensure that, even if there were some head movement during testing, the test targets would be presented within the expansion area. A total of 40 targets (two repetitions of 20 targets) were shown in each of the test zones. In addition, 40 targets (two repetitions of 20 targets) were presented outside of the test zones at arbitrary positions covering a wide area in the seeing and non-seeing hemi-fields (Figure 3). These targets ensured that the participants did not expect targets to appear in only specific areas of the visual field; they were not included in analyses of detection performance. Eight additional targets (two repetitions of 4 targets) to monitor fixation losses were presented in non-prism areas of the blind hemifield, close to the border with the seeing visual field; median fixation losses were 0% (interquartile range 0% to 4%).
Detection Test Conditions and Procedures
For each of the prism fittings (side of HH and opposite side), detection performance was evaluated on two backgrounds (complex patterned and uniform gray) and in two viewing conditions (prism-eye only and binocular). All of the tests for one fitting (usually two sessions) were completed before the prisms were swapped to the other eye. For each fitting, participants wore the prisms as much as possible every day for at least 2 weeks before testing (median 2.75 hours per day, range 1.25 to 18 hours). Order of backgrounds and viewing conditions were counterbalanced as far as possible across participants, but the order was the same for the two fittings for each participant.
During the detection tests participants were instructed to maintain fixation on the central cross and to press a hand-held response button, connected to the computer, whenever a target was seen. Participants practiced the task before experimental trials began and were allowed to take breaks whenever needed. Kinetic perimetry was used at the start of every test condition to ensure that the test zones were within the intended areas of the visual field. When necessary, participants were optically corrected for the 1m test distance either with study glasses prescribed for that distance or with clip-on +1D lenses.
Questionnaires and Clinical Decision
After wearing each prism fitting for at least 2 weeks, all participants completed a short questionnaire addressing their experiences of using that prism fitting.12 At the end of the study, preference for one prism fitting over the other was determined. Based on subjective preferences and quantitative detection data, a clinical decision regarding continued use of the prism glasses (and which side fitting) was made.
Data Analysis
Since the detection rate data were not normally distributed, even after a probit transform,29, 30 statistical analyses of group data were conducted on the raw percentages using the non-parametric Wilcoxon Signed Rank test. A significance level of α=0.05 was used.
RESULTS
Participants with normal binocular vision
Sensory dominance
The majority of participants with normal binocular vision (7 of 8) had no clear dominance; only one demonstrated consistent sensory ocular dominance on the test battery (same eye dominant on three of the four tests).
Side of prism fitting
For the 6 participants who completed testing with both fittings, we compared detection rates for binocular viewing when the prism was fitted in front of the eye corresponding to the side of HH and in front of the opposite eye. For the expansion area, there were no significant differences in detection rates between the two prism fittings for both the patterned background (Figure 4A) and the uniform gray background (p = 0.285 and 0.713, respectively). For the seeing hemifield area (Figure 4B), there were also no significant differences in detection rates between the two prism fittings for both backgrounds (p = 0.180 and 0.414, respectively). Therefore, subsequent data analyses were performed with the data collapsed across the two fittings for these 6 participants. In addition, data were included from the two participants who only completed testing for fitting on the side of the HH.
Figure 4.
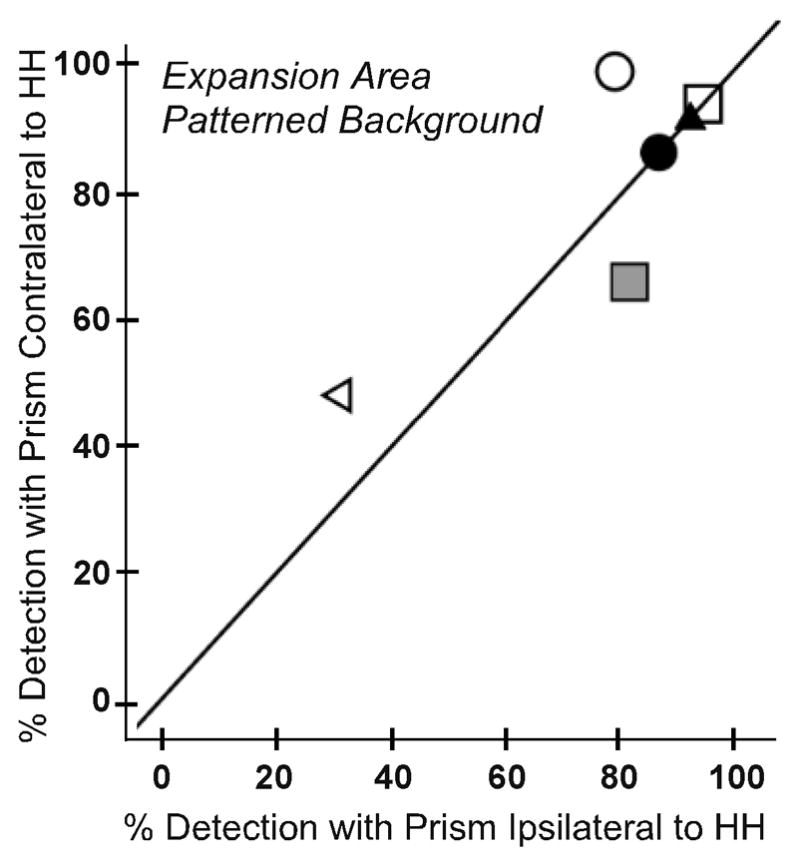
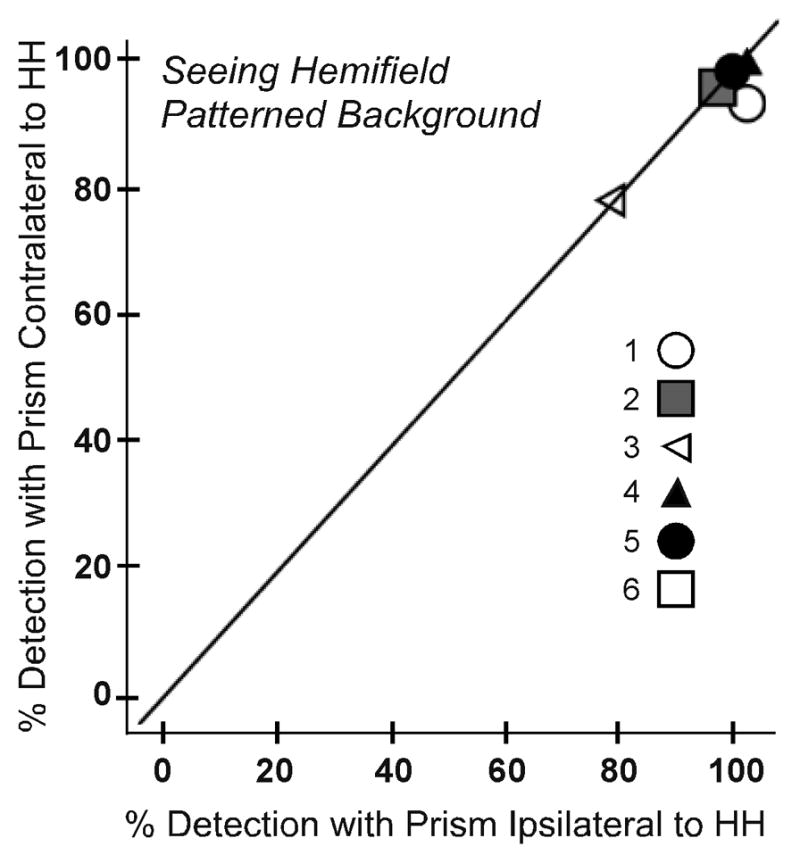
Detection rates for each participant under binocular viewing on the patterned background when peripheral prism segments were fit ipsilateral and contralateral to the side of HH: (A) prism expansion area test zone and (B) seeing hemifield test zone. In both test zones detection rates were not significantly different for the two fittings (p > 0.2). The y=x reference line is plotted to assist interpretation; data points falling on the reference line indicate no difference between the ipsilateral and contralateral fittings.
Seeing hemifield zone under the apical scotoma
As expected, for monocular prism-eye only viewing, very few targets were detected in the seeing hemifield zone under the apical scotoma; median detection rates were only 1% (IQR 0 % – 5%). However, in binocular viewing the fellow eye compensated for the monocular apical scotoma and detection rates in the main seeing hemifield zone and the seeing hemifield zone under the apical scotoma were not significantly different (p > 0.5). Only results for the main seeing hemifield zone are reported below.
Suppression of prism image under binocular viewing?
To evaluate whether there was decreased predominance or local suppression of the prism image, detection rates in the expansion test zone were compared for monocular and binocular viewing. As expected, there was no significant difference in detection between monocular and binocular viewing on the uniform gray background (p = 0.893; Figure 5A). However, contrary to our prediction, there was also no significant difference between monocular and binocular viewing on the patterned background (p = 0.483; Figure 5B). Median detection performance on the patterned background was 78% for monocular viewing (IQR 69–78) and 86% for binocular viewing (IQR 64–91).
Figure 5.
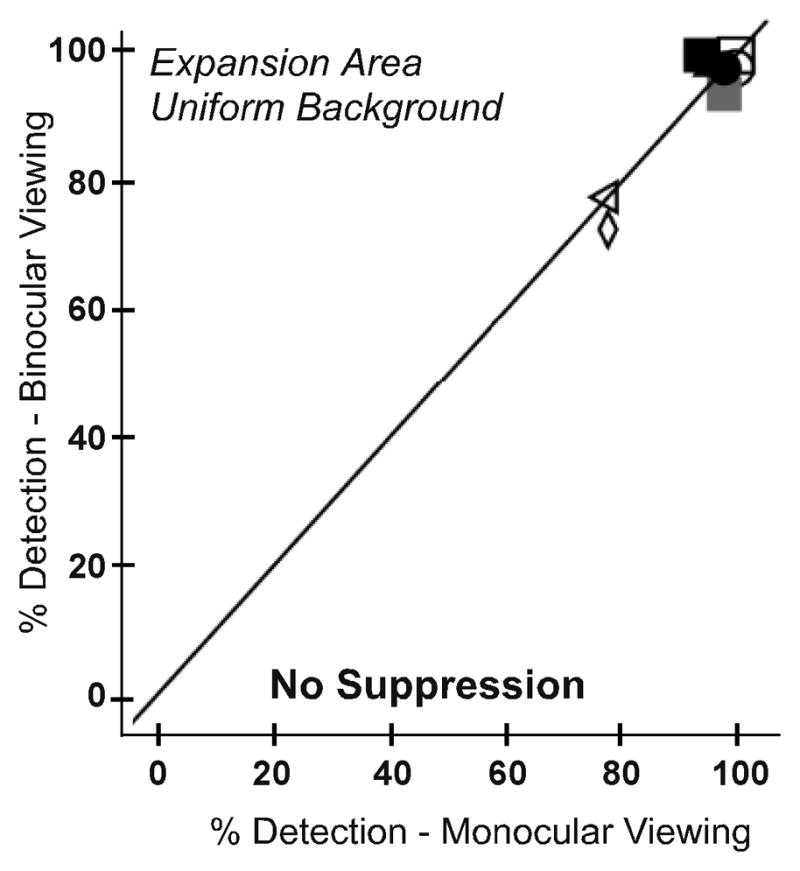
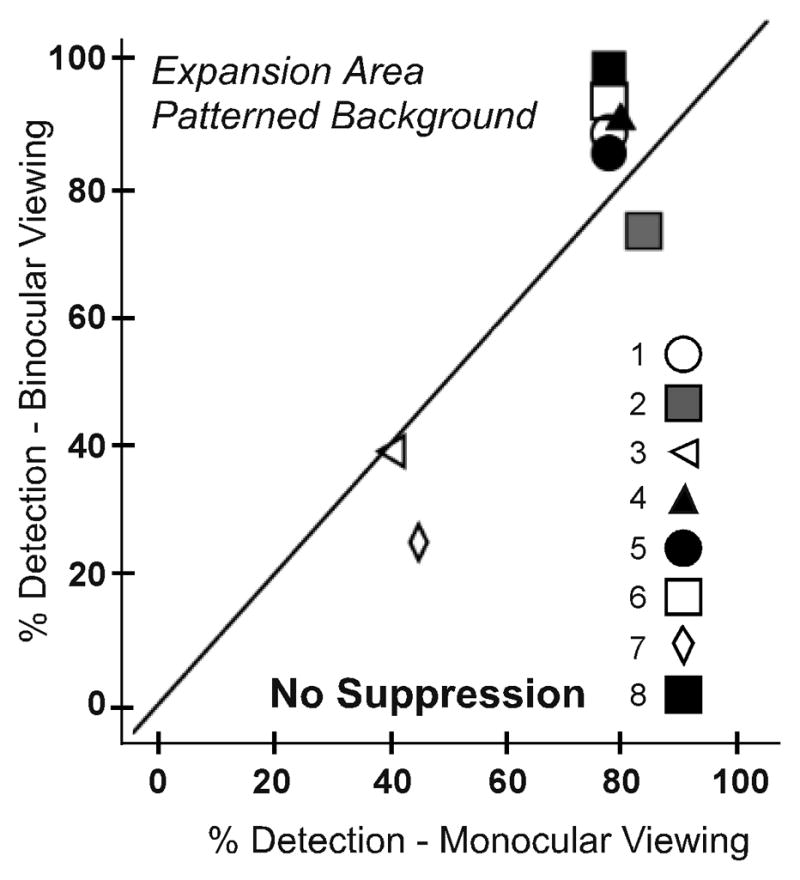
Detection rates for each participant in the expansion area test zone for monocular and binocular viewing for (A) the uniform and (B) the patterned background. There were no significant differences in detection rates between monocular and binocular viewing on either background, suggesting no reduction in predominance or local suppression of the prism image in binocular viewing.
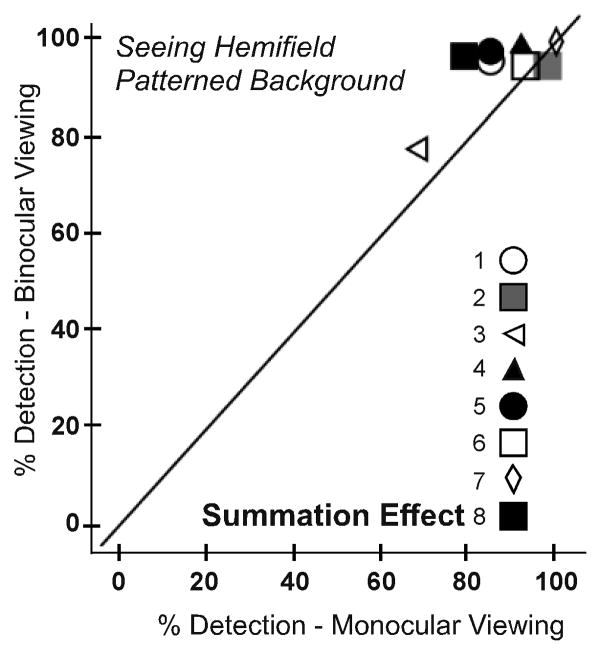
We did, however, find an effect of binocular summation in the seeing hemifield. While this effect was not observed with the uniform background (as expected; p = 0.246; Figure 6A), there were significantly higher detection rates for binocular than monocular viewing on the patterned background (p=0.043; Figure 6B).
Figure 6.
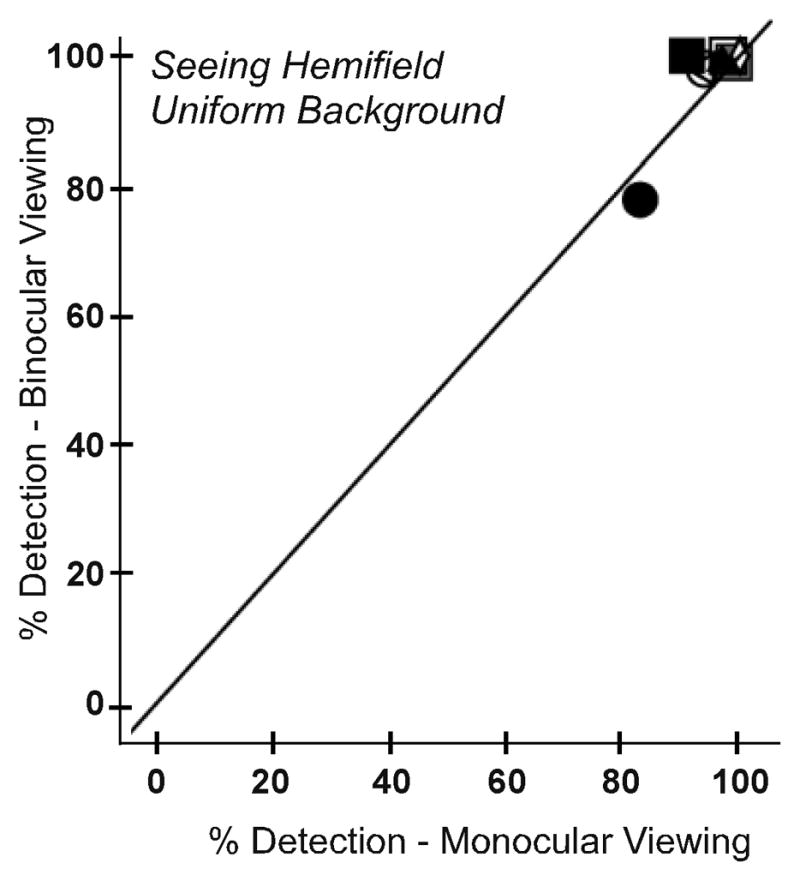
Detection rates for each participant in the main seeing hemifield area test zone for monocular and binocular viewing for (A) the uniform and (B) the patterned background. On the patterned background, detection was better under binocular than monocular viewing consistent with a binocular summation effect, which was not evident on the uniform background (as expected).
Effect of Background
As expected, detection rates for the patterned background were lower than for the uniform gray background. This trend was apparent in both the expansion and the seeing hemifield test zones for both monocular and binocular viewing. However, the reduction was greater in the prism expansion area (97% to 82%, p = 0.012; Figure 7A; data pooled across monocular and binocular viewing) than the seeing hemifield (97% to 93%, p = 0.175; Figure 7B). This difference was likely due to the degradation in image quality induced by the high-power Fresnel prism, as shown below.
Figure 7.
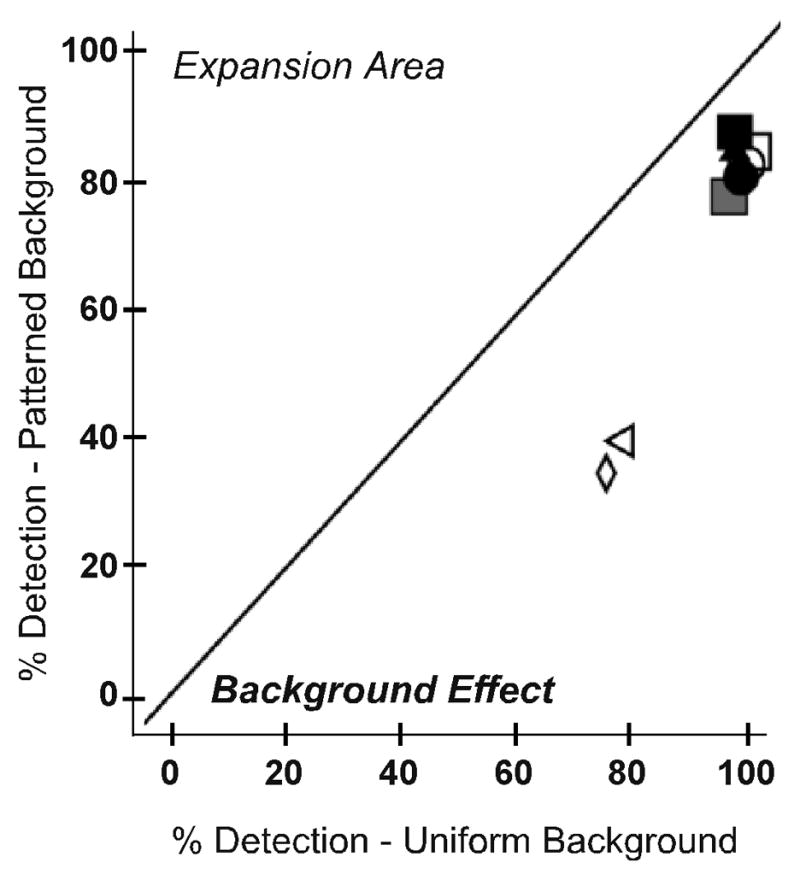
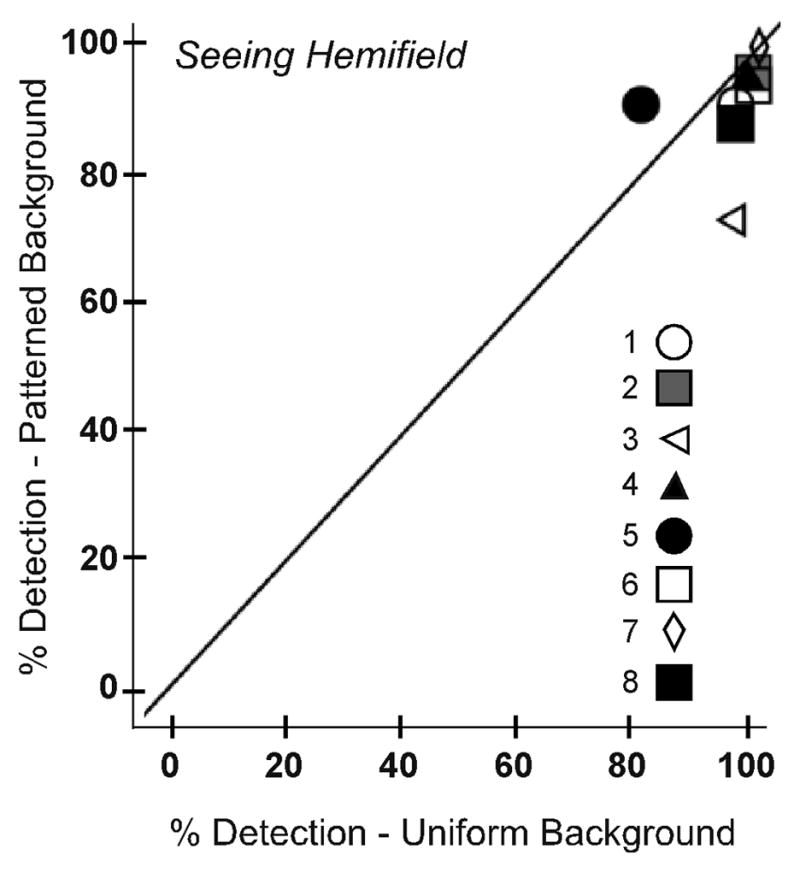
Detection rates for each participant on the uniform and patterned backgrounds for (A) the prism expansion area and (B) the seeing hemifield test zones. In the prism expansion area, detection rates were significantly lower on the patterned than the uniform gray background (p=0.012). Data are pooled across monocular and binocular viewing.
Effect of prism alone
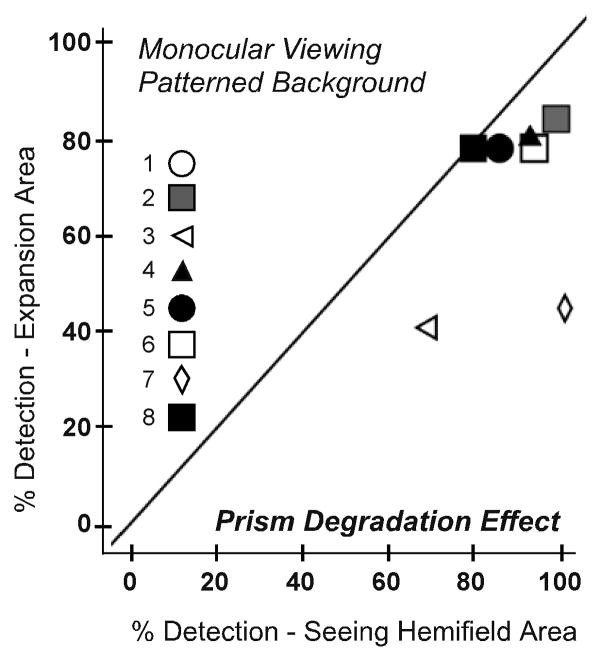
The effect of the prism alone on detection (without the possible confound of binocular rivalry) was examined by comparing detection rates in the expansion and seeing hemifield zones for monocular (prism-eye only) viewing. On the uniform gray background there was little difference in monocular detection rates between the expansion and seeing hemifield areas (median difference only 3%; Figure 8A). By comparison, on the patterned background, monocular detection in the prism expansion area was 10% (median) lower than in the seeing hemifield (p=0.012; Figure 8B).
Figure 8.
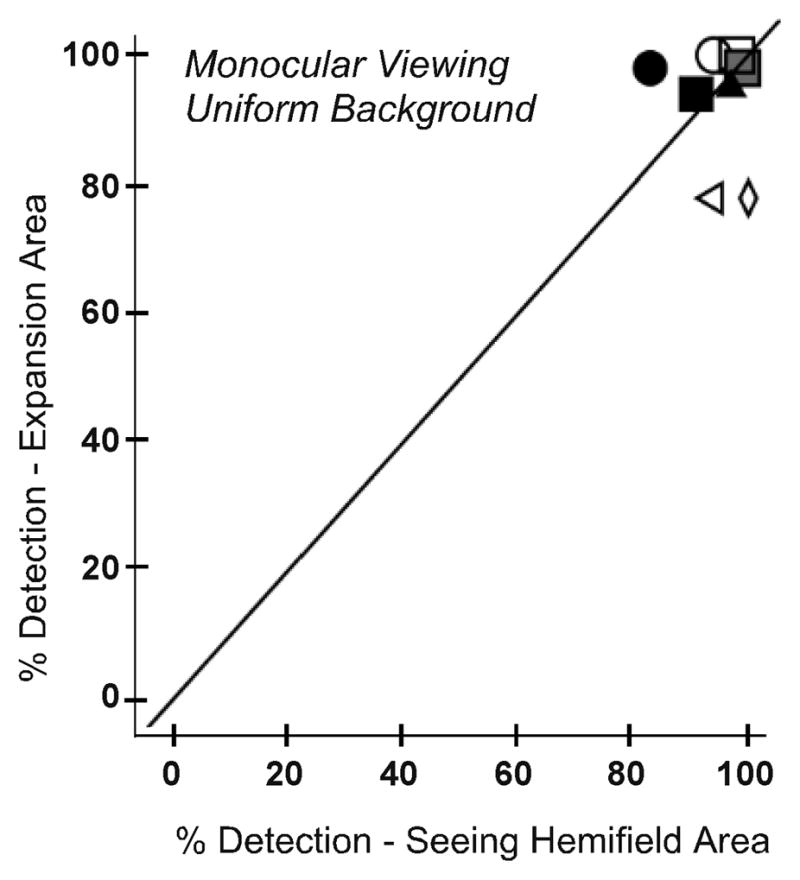
The degrading effect of the Fresnel prism was measured by comparing detection rates for each participant in the seeing hemifield and expansion area test zones under monocular viewing for (A) the uniform and (B) the patterned background. The poor optical quality of the prisms reduced detection by about 10% (median) on the patterned background, but had little effect on detection rates on the uniform background.
Long Term Wear and Clinical Decision
Of the 8 participants who participated in the main study, five continued to wear the prisms at the conclusion of the study (3 with complete HH and 2 with quadranopia; Table 1), reporting that they used the prism glasses mostly when moving about outdoors or in unfamiliar environments. Participants ranked the helpfulness of the prism glasses in these circumstances; the median reported score was 3 out of a possible score of 5 (somewhat to moderately helpful) for detecting obstacles on their blind side. Of the 6 participants who completed testing with both prism fittings, two continued wearing the peripheral prisms on the side of HH (1 current with complete HH and 1 new wearer with quadranopia) and 1 new wearer with complete HH continued wear on the opposite side.
Participants with Abnormal Binocular Vision and Strong Ocular Dominance
Two participants with HH, who did not meet the main study inclusion criteria for visual acuity, normal binocularity (and in one case, integrity of the seeing hemifield), were included as cases of strong ocular dominance. Participant 9 (left HH; age 95, had not worn peripheral prisms before the study) had significant ocular pathology (primary open angle glaucoma, and geographic atrophy) in the right eye only (VA 20/63), and consistently showed left eye (VA 20/32) preference on all dominance tests. Although there was glaucomatous visual field loss in the right eye, there was still sufficient remaining field to encompass the prism-shifted images from the blind hemifield when the prisms were fitted in front of that eye. However, there was insufficient remaining field to place a seeing hemifield test zone in an area outside of the apical scotoma where both the prism eye and the fellow eye would be able to detect targets in monocular and binocular conditions; therefore, only results for the expansion zone are reported. On the patterned background, detection in the prism expansion area was lower when the prism was fit before the non-dominant eye than the dominant eye; this difference was statistically significant31 in both monocular and binocular viewing (p=0.01 and p=0.0001, respectively; Figure 9). The same was also true for the gray background for binocular viewing (p=0.0001), but not monocular viewing (p=0.50). Consistent with these findings, this participant reported that the peripheral prism segments were helpful for detecting obstacles on the blind side when fitted in front of the dominant left eye, but less helpful when in front of the non-dominant eye. She reported they were especially helpful when navigating in church or in the shopping mall and requested to continue to wear the prism segments (on the dominant eye) in these situations at the conclusion of the study.
Figure 9.
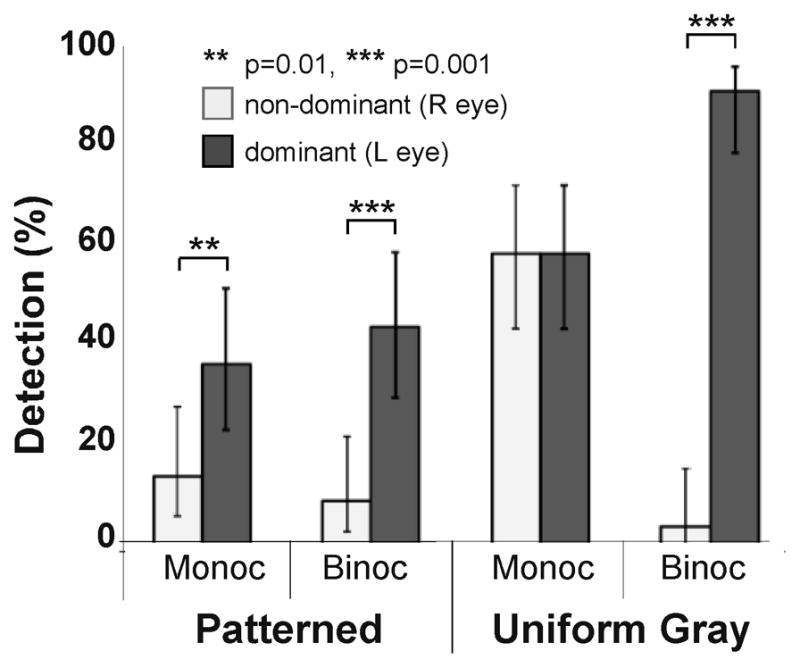
Detection performance in the expansion area for participant 9 with left HH and a strongly dominant left eye due to unilateral (right eye) ocular pathology. Detection rates were significantly higher in the prism expansion area when the prism was fit to the left than the right eye for both viewing conditions on the patterned background and for the binocular condition on the uniform background. Error bars are 95% confidence limits.
Participant 10 (left HH; age 79) with left small angle exotropia, reported crossed diplopia on the Worth 4 Dot test, and was right eye dominant on all other ocular dominance tests (OD 20/16 OS 20/60). This participant had worn peripheral prisms, fitted elsewhere, in front of the left, non-dominant eye for 6 years before participating in the study. Due to transportation difficulties, detection tests were completed for only the habitual (left-eye) prism fitting; we were unable to arrange subsequent visits to fit prisms in front of the right dominant eye. On the patterned background, monocular detection performance in the expansion zone for the left-eye prism-fitting was very low (25%), and significantly worse under binocular viewing (8%, p=0.0001; Figure 10), suggesting reduced predominance or partial local suppression of the prism image from the non-dominant eye. In the seeing hemifield on the patterned background, monocular (prism-eye only) detection was also reduced relative to binocular detection (p=0.0001), indicating overall poorer visual function in the prism eye. Detection on the gray background was higher for all viewing conditions with minimal differences between monocular and binocular viewing (p>0.1). After the study, this participant elected to continue wearing the prism glasses with the habitual left-eye prism fitting for occasional use when walking outside. However, he reported that the glasses were not helpful for detecting obstacles in crowded situations.
Figure 10.
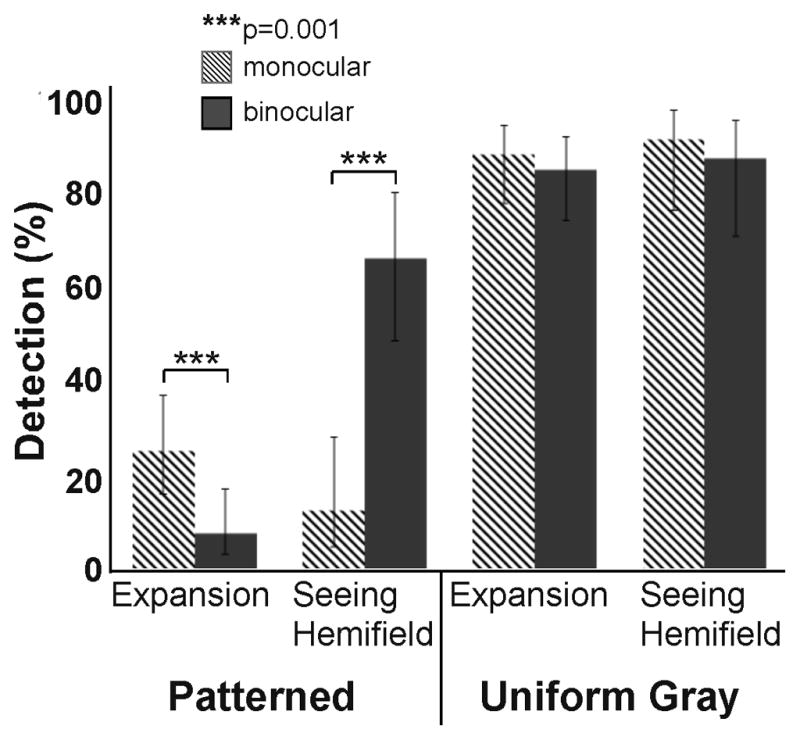
Detection rates when peripheral prisms were fit in front of the non-dominant left eye of participant 10 with left HH and left exotropia. On the patterned background, monocular (prism-eye only) detection was significantly reduced in both the expansion and seeing hemifield zones (compared to the binocular seeing hemifield condition) indicating overall poor visual function of the prism eye. In the expansion zone, detection rates on the patterned background were significantly lower in binocular than monocular viewing, suggesting partial local suppression of the prism image from the non-dominant prism eye. Error bars are 95% confidence limits.
DISCUSSION
Consistent with our predictions, for participants with normal binocularity, detection rates in the prism expansion area were similar in binocular and monocular viewing on both the patterned and uniform gray backgrounds. These findings suggest that there was no reduced predominance or local suppression of the prism image in binocular viewing even in the more complex, pattern-viewing condition where there was a stimulus for binocular rivalry.26 As expected, overall detection rates were lower on the patterned (masking) background than the uniform gray background, with a greater reduction in the prism-affected expansion area than the prism-free seeing hemifield. These results suggest that the reduction was caused by the contrast degradation and poor optical quality of the Fresnel prism images15 rather than by local suppression of the lower-contrast prism image on the more complex background.
Median detection rates were only 10% lower in the prism expansion area than the seeing hemifield zone on the patterned background in both monocular and binocular viewing which is promising for the utility of the prisms in real-world conditions. Nevertheless, there was a high degree of between-subject variability in detection rates for the prism expansion area (ranging from 25% to 98% for binocular viewing on the patterned background; Figure 5B). There were two participants with noticeably lower detection rates (participants 3 and 7 in Figure 5B). One was the participant who would not permit us to place new press-on prisms on his glasses; therefore detection rates may have been affected by deterioration in the optical quality of the old prisms. The other participant had a moderate myopic prescription (spherical equivalent OD −4.87 D, OS −5.25 D) and used permanent prism glasses without any refractive correction in the area of the lens occupied by the prism segments that may have contributed to the lower detection rates.
In agreement with previous studies,19, 20 the majority of participants with normal binocular vision demonstrated mixed results on sensory ocular dominance testing, exhibiting no truly dominant eye, and little overall difference in detection rates when prisms were fitted on the side of the HH or the opposite side. However, as expected, strong ocular dominance did affect detection performance in the prism expansion area (and the seeing hemifield area). Participant 9, with strong left-eye dominance, demonstrated significantly decreased monocular and binocular detection in the expansion area when the prism segments were fit to the non-dominant right eye as opposed to the dominant left eye. Participant 10, who only wore prisms on the non-dominant eye, exhibited significantly reduced detection in the expansion area in binocular compared to monocular viewing on the complex background. This may be indicative of reduced predominance (or partial local suppression) of the expansion area under binocular viewing.
Our results suggest that for patients with normal binocularity and without strong ocular dominance, peripheral prisms can be fitted in front of either eye. However, fitting in front of the eye on the side of the hemianopia is generally recommended, especially if fitting press-on segments that extend across the entire lens, as in the original design (Figure 2 in Peli11 or Figure 1 in Bowers et al.12), rather than the smaller, pre-cut segments that are now widely used (Figure 1). If prism segments that extend across the whole lens are fitted in front of the eye on the side opposite to the HH, there is a possibility that the optical (apical) scotoma caused by the prism could reduce the temporal visual field as the apex of the prism will be in the monocular temporal crescent region of the field and thus will not be compensated by the other eye’s field.28 However, if a prism segment spanning the full width of the lens is fitted in front of the eye on the side of the HH, the prism apex will be placed nasally and the apical scotoma will likely be compensated by the normal hemifield of the other eye. With both the smaller 22mm-wide pre-cut press-on and permanent segments, the apical scotoma usually falls within the area of the binocular overlap of the seeing hemifield and is compensated for by the non-prism eye, as was the case in this study.
For patients with abnormal binocularity and/or strong ocular dominance, the likelihood of reduced predominance or local suppression of the prism image needs to be considered. Therefore preference should be given to fitting peripheral prisms in front of the dominant eye, while still considering the possible impact of the apical scotoma.28 It is also important to ensure that the eye in front of which the prism is fitted has sufficient remaining visual field to encompass the prism-shifted images from the blind hemifield.
This study is a first step in evaluating detection performance with peripheral prism glasses using more complex patterned backgrounds than the uniform backgrounds typically used in conventional perimetry. The results from participant 10 clearly demonstrate the advantage of using a rivalrous background rather than a plain uniform gray background when the goal of the perimetry assessment is to predict functional performance in real world situations. On the uniform gray background, detection rates were similar in the prism expansion area and the seeing hemifield for both monocular and binocular viewing. However, on the patterned background, there was evidence of reduced predominance (or local suppression) of the prism image in binocular viewing.
Our results suggest that, in binocular viewing conditions, neither reduced predominance nor exclusive local suppression of the prism expansion area occur for patients with normal binocularity. Comparable results were recently reported for a similar paradigm, but using natural image backgrounds rather than patterned backgrounds.32 These findings are confirmatory of the subjective reports of the utility of the peripheral prism glasses in real world situations.4, 11–13 In future studies we will increase the reality of the test conditions to bring them closer to those experienced in everyday dynamic environments by using motion video backgrounds.32
Acknowledgments
Financial Support: Supported by National Institutes of Health grants EY12890 (EP), EY018680 (ARB), and 2P30EY003790.
Footnotes
Financial Disclosure: Dr. Peli has patent rights (assigned to Schepens Eye Research Institute) for the use of the peripheral prisms (licensed to Chadwick Optical).
References
- 1.Gilhotra JS, Mitchell P, Healey PR, Cumming RG, Currie J. Homonymous visual field defects and stroke in an older population. Stroke. 2002;33:2417–20. doi: 10.1161/01.str.0000037647.10414.d2. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Kedar S, Lynn MJ, Newman NJ, Biousse V. Homonymous hemianopias: clinical-anatomic correlations in 904 cases. Neurology. 2006;66:906–10. doi: 10.1212/01.wnl.0000203913.12088.93. [DOI] [PubMed] [Google Scholar]
- 3.Peli E, Peli D. Driving With Confidence: A Practical Guide to Driving With Low Vision. Singapore: World Scientific; 2002. [Google Scholar]
- 4.O’Neil EC, Connell PP, O’Connor JC, Brady J, Reid I, Logan P. Prism Therapy and Visual Rehabilitation in Homonymous Visual Field Loss. Optom Vis Sci. 2011;88:263–8. doi: 10.1097/OPX.0b013e318205a3b8. [DOI] [PubMed] [Google Scholar]
- 5.Vu H, Keeffe J, McCarty C, Taylor H. Impact of unilateral and bilateral vision loss on quality of life. Br J Ophthalmol. 2005;89:360–3. doi: 10.1136/bjo.2004.047498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warren M. Pilot Study on Activities of Daily Living Limitations in Adults with Hemianopsia. American Journal of Occupational Therapy. 2009;23:626–33. doi: 10.5014/ajot.63.5.626. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JM. An overview of enhancement techniques for peripheral field loss. J Am Optom Assoc. 1993;64:60–70. [PubMed] [Google Scholar]
- 8.Cohen JM, Waiss B. Visual field remediation. In: Cole RG, Rosenthal BP, editors. Remediation and Management of Low Vision. St. Louis: Mosby; 1996. pp. 1–25. [Google Scholar]
- 9.Cotter SA. Uses of Prism in Low Vision. In: Weiss NJ, Brown WL, editors. Clinical Uses of Prism; A Spectrum of Applications. St. Louis: Mosby-Year Book, Inc; 1995. pp. 279–300. [Google Scholar]
- 10.Young CA. Homonymous hemianopsia during pregnancy aided by reflecting prism. Arch Ophthalmol. 1929;2:560–5. [Google Scholar]
- 11.Peli E. Field expansion for homonymous hemianopia by optically-induced peripheral exotropia. Optom Vis Sci. 2000;77:453–64. doi: 10.1097/00006324-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Bowers AR, Keeney K, Peli E. Community-based trial of a peripheral prism visual field expansion device for hemianopia. Arch Ophthalmol. 2008;126:657–64. doi: 10.1001/archopht.126.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giorgi RG, Woods RL, Peli E. Clinical and laboratory evaluation of peripheral prism glasses for hemianopia. Optom Vis Sci. 2009;86:492–502. doi: 10.1097/OPX.0b013e31819f9e4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tant M. Vision 2008-The 9th International Conference on Low Vision. Montreal, Canada: International Society for Low-Vision Research and Rehabilitation (ISLRR); 2008. Do peripheral prism visual field expansion glasses assist drivers with hemianopia (abstract) [Google Scholar]
- 15.Katz M. Contrast sensitivity through hybrid diffractive, Fresnel, and refractive prisms. Optometry. 2004;75:509–16. doi: 10.1016/s1529-1839(04)70176-1. [DOI] [PubMed] [Google Scholar]
- 16.Blake R. A primer on binocular rivalry, including current controversies. Brain and Mind. 2001;2:5–38. [Google Scholar]
- 17.Blake R, O’Shea RP, Mueller TJ. Spatial zones of binocular rivalry in central and peripheral vision. Vis Neurosci. 1992;8:469–78. doi: 10.1017/s0952523800004971. [DOI] [PubMed] [Google Scholar]
- 18.Handa T, Uozato H, Higa R, Nitta M, Kawamorita T, Ishikawa H, Shoji N, Shimizu K. Quantitative measurement of ocular dominance using binocular rivalry induced by retinometers. J Cataract Refract Surg. 2006;32:831–6. doi: 10.1016/j.jcrs.2006.01.082. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Lam CS, Yu M, Hess RF, Chan LY, Maehara G, Woo GC, Thompson B. Quantifying sensory eye dominance in the normal visual system: a new technique and insights into variation across traditional tests. Invest Ophthalmol Vis Sci. 2010;51:6875–81. doi: 10.1167/iovs.10-5549. [DOI] [PubMed] [Google Scholar]
- 20.Seijas O, Gomez de Liano P, Gomez de Liano R, Roberts CJ, Piedrahita E, Diaz E. Ocular dominance diagnosis and its influence in monovision. Am J Ophthalmol. 2007;144:209–16. doi: 10.1016/j.ajo.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 21.Gauthier L, Dehaut F, Joanette Y. The Bells Test: A quantitative and qualitative test for visual neglect. Int J Clin Neuropsychol. 1989;11:49–54. [Google Scholar]
- 22.Schenkenberg T, Bradforn DC, Ajax ET. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology. 1980;30:509–17. doi: 10.1212/wnl.30.5.509. [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive status of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Peli E. The optical functional advantages of an intraocular low-vision telescope. Optom Vis Sci. 2002;79:225–33. doi: 10.1097/00006324-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Woods RL, Apfelbaum HL, Peli E. DLP-based dichoptic vision test system. J Biomed Opt. 2010;15:1–13. doi: 10.1117/1.3292015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doherty AL, Bowers AR, Luo G, Peli E. Object detection in the ring scotoma of a monocular bioptic telescope. Arch Ophthalmol. 2011;129:611–7. doi: 10.1001/archophthalmol.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Field DJ. Relations between the statistics of natural images and the response properties of cortical cells. J Opt Soc Am A Opt Image Sci Vis. 1987;4:2379–94. doi: 10.1364/josaa.4.002379. [DOI] [PubMed] [Google Scholar]
- 28.Ross NC, Bowers AR, Peli E. Consideration of optical scotomas in designing visual field expansion devices. Invest Ophthalmol Vis Sci. 2009;50 ARVO E-abstract 4734. [Google Scholar]
- 29.Pearson K. On the Theory of Contingency and Its Relation to Association and Normal Correlation. Cambridge University Press; London: 1904. [Google Scholar]
- 30.Winer BJ, Brown DR, Michels KM. Statistical Principles in Experimental Design. McGraw-Hill; New York: 1991. [Google Scholar]
- 31.Newcombe RG, Altman DG. Proportions and their differences. In: Altman DG, Machin D, Bryant TN, Gardner MJ, editors. Statistics with confidence. London, UK: British Medical Journal Publishing Group; 2006. pp. 45–56. [Google Scholar]
- 32.Shen J, Peli E, Bowers AR. Effect of motion on detection with unilateral peripheral prisms for hemianopia. Invest Ophthalmol Vis Sci. 2011;52 ARVO E-Abstract 390. [Google Scholar]