Fig. 2.
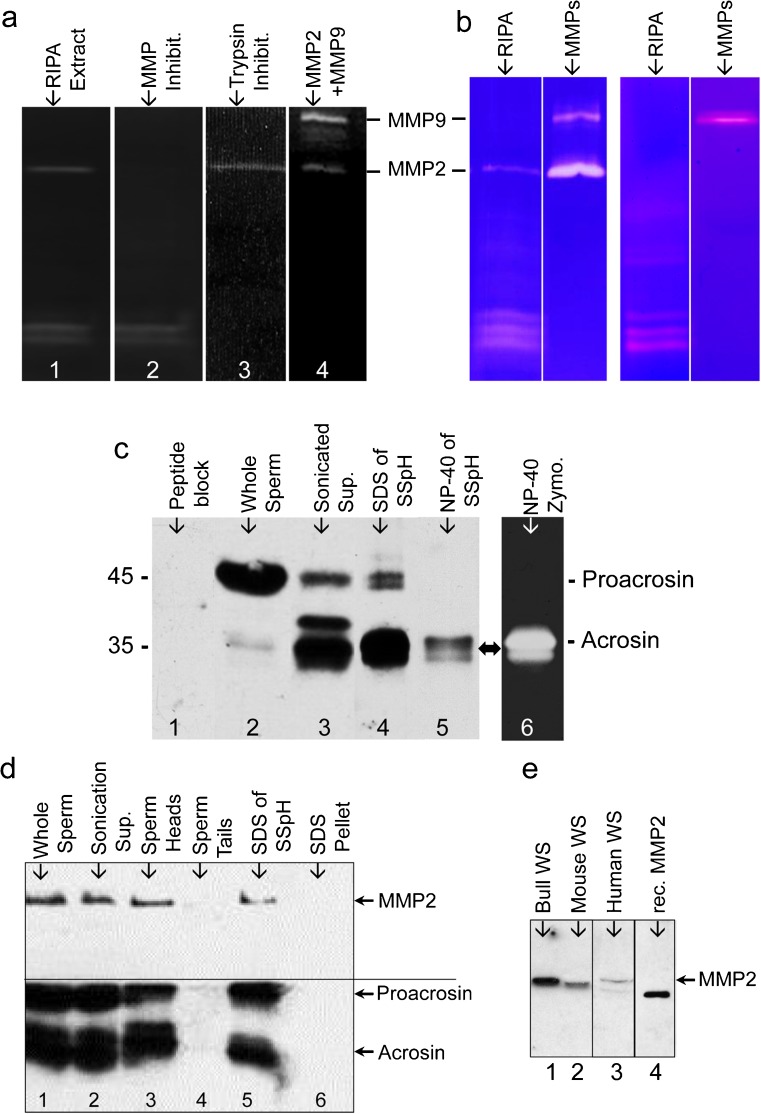
a Gelatin zymograms of detergent extracts (RIPA ) of SSpH reveal MMP and serine proteinases as components of the IAM. Blockage of the 72-kDa enzymatic activity with GM6001 (Ilomastat) (lane 2) indicates a metalloprotease and blockage of the 35-kDa enzymatic activities by trypsin inhibitor (lane 3) indicates serine proteases. Lane 4, a positive control, is loaded with a trophoblast cell medium containing both MMP2 (72 kDa) and MMP9 (92 kDa). b Gelatin zymogram without (control) and with (block) a cyclic disulfide bonded peptide (CTTHWGFTLC) on RIPA extracts of SSpH (lane 1) and trophoblast media (lane 2), containing both MMP2 and MMP9 enzymatic activities. c Immunoblotting verification that acrosin is responsible for serine protease activity in detergent extracts of SSpH. Anti-acrosin antibody detects proacrosin in western blots of whole bull epididymal sperm (lane 2) and is blocked when preincubated with the peptide it was raised against (lane 1). Sonication of whole sperm causes proacrosin cleavage into its active forms as indicated both in the resultant sonication supernatant (lane 3) and in SSpH (lane 4). The 2 % NP-40 (non-ionic detergent) extract of SSpH (lane 5) is less efficient in stripping SSpH of proacrosin/acrosin than 2 % SDS (lane 4). Lane 6 is a gelatin zymogram of the NP-40 extract loaded in lane 5, confirming that the enzymatic activities found at the 35-kDa level are due to acrosin. d Immunoblotting verification that both MMP2 and Proacrosin/Acrosin are constituents of the sonicated bull sperm head. Freeze–thawed sperm (lane 1) were sonicated and separated by centrifugation into three fractions: supernatant (lane 2), SSpH (lane 3), and tails (lane 4). The sperm heads were then extracted with SDS (lane 5) and compared with pellet (lane 6). The upper part of the western blot was probed with anti-pMMP2, while the bottom part below the demarcating line was probed with polyclonal anti-bull acrosin antibody. e Immunoblots probed with anti-tMMP2 showing that MMP2 is present in bull, mouse and human spermatozoa (WS). Human recombinant MMP2, minus the pre-domain, is used as a positive control (rec. MMP2)