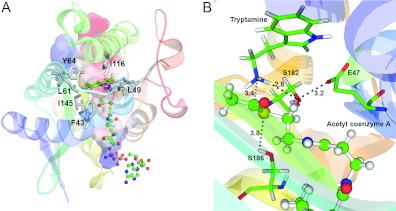
Figure 4. A ternary-docking model.
(A) Tryptamine was docked into the substrate-binding cavity of the Dat21–230–AcCoA complex by GOLD [29,50]. The enzyme is shown as a ribbon diagram. The surface electrostatic potential representation of the cavity is shown. Tryptamine is shown as a stick model. Non-polar residues are shown as grey stick models. AcCoA is shown as a ball-and-stick model. Both substrate and cofactor are within the cavity. (B) The catalytic triad residues of Dat. The tryptamine and catalytic triad residues are represented as stick models. AcCoA is shown as a ball-and-stick model. The broken lines identify interactions between the catalytic residues, tryptamine and AcCoA. The numbers represent distance separations between various atoms in angstroms.