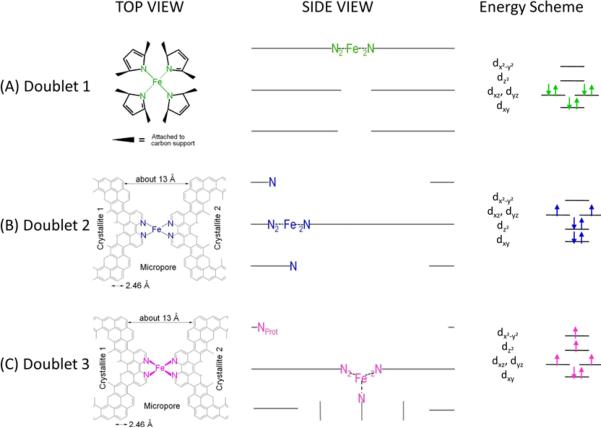
Figure 2.
Side views and top views of the proposed structures of: (A) the FeN4/C catalytic site in heat-treated, macrocycle-based catalysts assigned to Mössbauer doublet D1, (B) the FeN2+2-like micropore-hosted site found in the catalyst prepared with iron acetate and heat-treated in ammonia assigned to doublet D2, and (C) the N-FeN2+2-like composite site, where N-FeN2+2 is assigned to doublet D3. In all side views, graphene planes are drawn as lines. In (B), the distance between the two nitrogen atoms belonging to the graphene planes above and below the FeN2+2-plane is similar to that in crystaline iron phthalocyanine (see Figure S1), thus similarly influencing the lattice contribution to the quadrupole splitting. In (C), the ironII ion in N-FeN2+2 is coordinated by five pyridinic nitrogen atoms, one of them belonging to a plane located at the vertical below the N4-plane. This axial nitrogen coordination moves the ironII ion out of the N4-plane towards the fifth coordinating nitrogen atom. Doublet 3 may exist as N-FeN2+2/C or as a composite site N-FeN2+2…Nprot/C, where Nprot is a protonable pyridinic nitrogen atom appearing at the edge of the upper plane in the side view of D3.28 Nprot is too far from NFeN2+2 to have an influence on the Mössbauer spectrum of D3, but it is close enough to drastically improve the turn-over frequency of the composite site proposed in (C).
Figure 2 also shows the energy schemes of D1, D2, and D3, as well as the filling of the molecular orbitals for the ironII ion in the structure proposed for each site according to its proposed spin state: low in D1, intermediate in D2, and high in D3.47 Part (D) of Figure 2 schematizes the changing ORR-activity of the composite N-FeN2+2…prot/C site, where N-FeN2+2/C has the D3 signature; the activity of the composite site is low when the basic N-group neighbouring the N-FeN2+2/C moiety is unprotonated (left) or protonated and anion-neutralized (right). The activity is high when this N-functionality is protonated but not neutralized (center).28