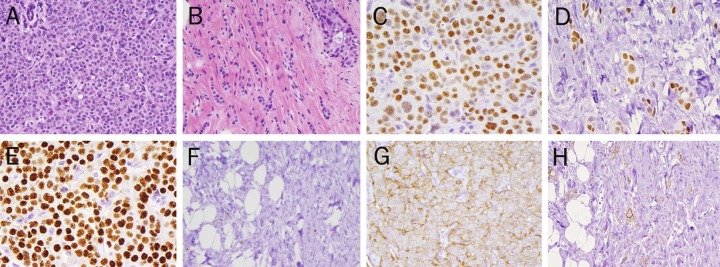
Figure 1.
ER, PR, and HER2 IHC Pre- and Post- Neoadjuvant Chemotherapy. A. Pre-treatment core biopsy, infiltrating ductal carcinoma, Grade II, with lobular features (40x, H&E) B. Post-neoadjuvant chemotherapy, residual infiltrating tubulolobular carcinoma, Grade I (40x, H&E) C. Pre-treatment Estrogen Receptor (ER) IHC: Positive (3+, 90%) (40x) D. Post-treatment Estrogen Receptor (ER) IHC: Positive (3+, 90%) (40x) E. Pre-treatment Progesterone Receptor (PR) IHC: Positive (3+, 90%) (40x) F. Post-treatment Progesterone Receptor (PR) IHC: Negative (<10%) (40x) G. Pre- treatment Her2 IHC: Positive (3+, 80%) (40x) H. Post-treatment Her2 IHC: Positive (3+, 50%) (40x).