Abstract
Langerhans cell histiocytosis (LCH) has a challenging and still unclear pathogenesis. A body of literature points to impaired maturation of the lesional dendritic cells, and to immune dysregulation in the form of increased FoxP3 cells. Various cytokine abnormalities such as expression of transforming growth factor (TGF)-β have been reported, as well as abnormalities in lipid content in LCH cells. Morphoproteomic techniques were applied to identify the signal transduction pathways that could influence histogenesis and immune regulation in osteolytic LCH. Five pediatric cases of osteolytic LCH were examined, using antibodies against CD1a, S100, CD68, CD8, FoxP3, phosphorylated (p)-STAT3 (Tyr705), protein kinase C (PKC)-α, phospholipase (PL)D1, fatty acid synthase (FASN), and zinc finger protein, Gli2. Positive and negative controls were performed. A FoxP3(+)/CD8(+) cell ratio was calculated by counting the FoxP3+ and CD8+ cells in 10 high power fields for each case. There is induction of sonic hedgehog (SHH) mediators consistent with TGF-β signaling pathway through Smad3-dependent activation of Gli2, findings supported by the plasmalemmal and cytoplasmic expression of PKC-α and PLD1, and nuclear expression of Gli2, in lesional cells. The FoxP3+/CD8+ cell ratio is increased, ranging from 1.7-7.94. There is moderate cytoplasmic expression of FASN in most of the Langerhans cells, a finding that supports previously published phospholipid abnormalities in LCH and is consistent with PKC-α/PLD1/TGF-β signaling. With our study, we strongly suggest that the TGF-β cell signaling pathway is a major player in the pathogenesis of LCH, leading to non-canonical induction of nuclear Gli2 expression, thereby contributing to osteoclastogenesis in LCH histiocytes. It could also cause a state of immune frustration in LCH, by inducing the transformation of CD4(+)CD25(-) cells into CD4(+)/FoxP3(+) cells. This coincides with the clinical evidence of a response to thalidomide in patients with osteolytic LCH, given its reported ability to reduce TGF-beta 1 and FoxP3 cells. Such TGF-β signaling in osteoclastogenesis and immune dysregulation, and the presence of FASN in the majority of cells, have additional therapeutic implications for osteolytic LCH.
Keywords: Morphoproteomics, TGF-β, signaling pathway, osteoclastogenesis, histogenesis, Langerhans cell histiocytosis
Introduction
Though Langerhans cell histiocytosis (LCH) is a disease mostly found in the pediatric population, it can also appear in adults. Distinct entities have been identified based on specific patterns of disease (monostotic and polyostotic). Approximatively two thirds of the children with LCH have single-system disease (monostotic or polyostotic lytic lesions of the bone, especially of the cranium) [1,2].
Currently there are two main theories regarding the pathogenesis of LCH. There is a body of literature suggesting that it is a clonal disease. Arico et al. [3] studied the incidence of Langerhans cell histiocytosis in presumed (p) monozygotic (MT) and dizygotic (DT) twins in which one of the siblings was affected by the disease. He observed that four out of five sets of pMT developed the disease at close intervals, and had similar manifestations. One of the 3 DT observed developed the disease. In a study of 72 cases of LCH, Da Costa and colleagues [4] showed using immunochemistry that there is consistent p53 expression implying a mutation, but at that point the authors did not find a recurrent genetic abnormality. Specifically, they performed sequencing of exons 5 to 8 of the p53 gene and found no alterations in 7 cases analyzed. In 2010, Badalian-Very and co-workers [5] reported that thirty-five out of sixty-one studied cases of LCH (adult and pediatric population) had a recurrent BRAF V600E mutation. Seventeen out of the twenty-five (65.38%) pediatric cases of osteolytic LCH (eosinophilic granuloma) with only bone involvement had the mutation. However, phosphorylated (p)-extracellular signal-regulated kinase (ERK) and p-mitogen-activated protein kinase (MAP)-ERK Kinase (MEK) were expressed in all pediatric and adult cases, suggesting that there must be alternative mechanisms contributing to the pathogenesis of this disease.
Moreover, it is difficult to explain a pure clonal origin for a disease that, in some cases, regresses spontaneously and in other cases has a very aggressive behavior. Therefore, many researchers have concentrated on the study of the immunophenotype and chemokine expression of lesional cells in LCH. Fleming et al. [6] demonstrated the aberrant co-expression of CCR6 and CCR7 in twenty-four cases of LCH. Geissmann and colleagues [2], in an attempt to characterize the LCH cells, showed that these cells do not express CD83 and CD-Lamp (markers of mature dendritic cells), but that the majority of cells express CD14 (marker of immature dendritic cells). Senechal and co-workers reported on an expansion of FoxP3 regulatory T cells in patients with LCH raising the possibility of dysregulation of the host immune system compromising its ability to eliminate LCH cells [7]. Additionally, osteoclasts, a consistent component of osteolytic LCH are regarded as a major contributor to osteolysis and the histogenesis of the osteolytic form of the disease.
Because of these existing data that support both clonal and dysmaturation/immune dysregulation-associated processes in the etiopathogenesis of osteolytic LCH, we decided to focus on defining the factors common to its histogenesis. In this context, we were guided by therapies with proven efficacy in osteolytic LCH, namely aminobisphosphonates that inhibit osteoclastic giant cells [8-14] and thalidomide, which inhibits transforming growth factor (TGF)-β signaling and downregulates T regulatory cells [15-19]. This focus is underscored by the previous report of Brown [20], noting that the latency-associated peptide of TGF-β1 and osteoclastogenic interleukin (IL)-11[21], a downstream effector of the TGF-β signaling pathway [22] are expressed in osteolytic LCH. Thus, the objectives of this study are two-fold: firstly, to identify additional components of the TGF-β signaling pathway involved in osteoclastogenesis and T regulatory cell expansion in osteolytic LCH using morphoproteomics; and secondly, to develop possible therapeutic strategies that target these histogenetic processes.
Methods
With Institutional Board Review approval, four pathologists from the University of Texas–Health Science Center analyzed formalin-fixed, paraffin-embedded tissue from five pediatric cases (age ranging from 2-12) of LCH osteolytic lesions (Table 1). Histologic (hematoxylin&eosin; H&E) and immunohistochemical (S100 [CMC715, Cell Marque, Rocklin, CA], CD1a [ab708, Abcam], and CD68 [M0814 Dako]) studies were performed on all cases. Monoclonal antibodies against phospholipase D1 (PC-PLD1, 28314, Santa Cruz Biotechnology, Santa Cruz), protein kinase C-α (PKC-α, 8393 Santa Cruz), phosphorylated (p)-signal transducer and activator of transcription (STAT)3 phosphorylated on tyrosine 705 (8059 Santa Cruz), zinc finger protein Gli2 (ab26056 abCam), fatty acid synthase (FASN) (3180 Cell Signaling Technology), FoxP3 (ab10563, Abcam), and CD8 (ab4055, Abcam) were applied after tissue rehydration and antigen-retrieval . Using bright-field microscopy, the expression or absence of PLD1, PKC-α, p-STAT3 (Tyr705), Gli2, and FASN was assessed for each case, along with the subcellular compartmental distribution of these individual monoclonal antibodies. Positive and negative controls were run concurrently.
Table 1.
Study Population of Pediatric Cases with Osteolytic LCH.
| Case | Age | Sex | Type of lesion |
|---|---|---|---|
| 1. | 3 yo | M | Monostotic; left sphenoid wing |
| 2. | 9 yo | M | Monostotic; left iliac crest |
| 3. | 12 yo | F | Monostotic; pterygoid process |
| 4. | 5 yo | F | Monostotic, right fibular lesion |
| 5. | 2 yo | F | Polyostotic; palate and skull lesions; skin involvement present |
A FoxP3 (+)/CD8 (+) cell ratio was calculated by counting the FoxP3 (+) and CD8 (+) cells in 10 high power fields for each case.
Results
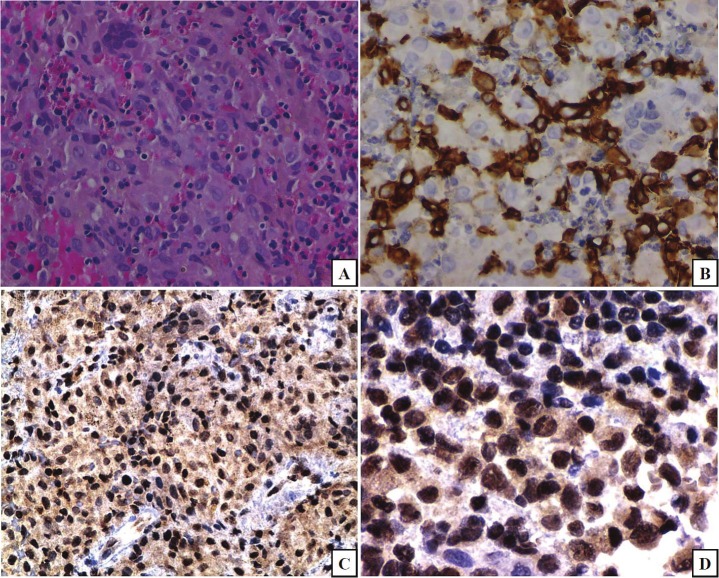
H&E evaluation of the tissue from the five cases examined showed destructive lesions comprised of a histiocytic population of cells with cleaved nuclei and nuclear grooves, admixed with multinucleated giant cells, including osteoclasts, and with an eosinophilic infiltrate. The histiocytic cells expressed S100, CD1a, and CD68 Figure 1 depicts H&E and CD1a; S100 and CD68 although expressed are not depicted]. A diagnosis of osteolytic Langerhans histiocytosis was rendered for each of the five cases, based on the above findings. The zinc finger protein Gli2 was variably expressed in the majority of the nuclei of the lesional cells (Figure 1, frames C and D) and has implications in osteoclastogenesis (vide infra).
Figure 1.
Hematoxylin-eosin (H&E) shows a proliferation of histiocytic cells, some with cleaved nuclei, nuclear grooves, admixed with multinucleated giant cells, including osteoclasts, and with an eosinophilic infiltrate (frame A). The lesional cells express CD1a (frame B). This pattern of expression is consistent with a diagnosis of Langerhans cell histiocytosis. Morphoproteomic application of a probe for glioma-associated oncogene protein (Gli)2 reveals nuclear expression in a majority of lesional Langerhans cells (frames C and D;DAB [brown] chromogen) with implications for TGF-β signaling and osteoclastogenesis. (original magnifications, x400 for A, B, and D and x200 for C).
The majority of lesional Langerhans cells had moderate-intense cytoplasmic and plasmalemmal expression of PKC-α (Figure 2A) as well as mild-to-moderate expression of PLD1 (Figure 2B), with similar compartmentalization. There was focal mild cytoplasmic expression of these protein analytes in the multinucleated giant cells. FASN had moderate, variable cytoplasmic expression in the Langerhans cells and somewhat stronger expression in the multinucleated giant cells (Figure 2C).
Figure 2.
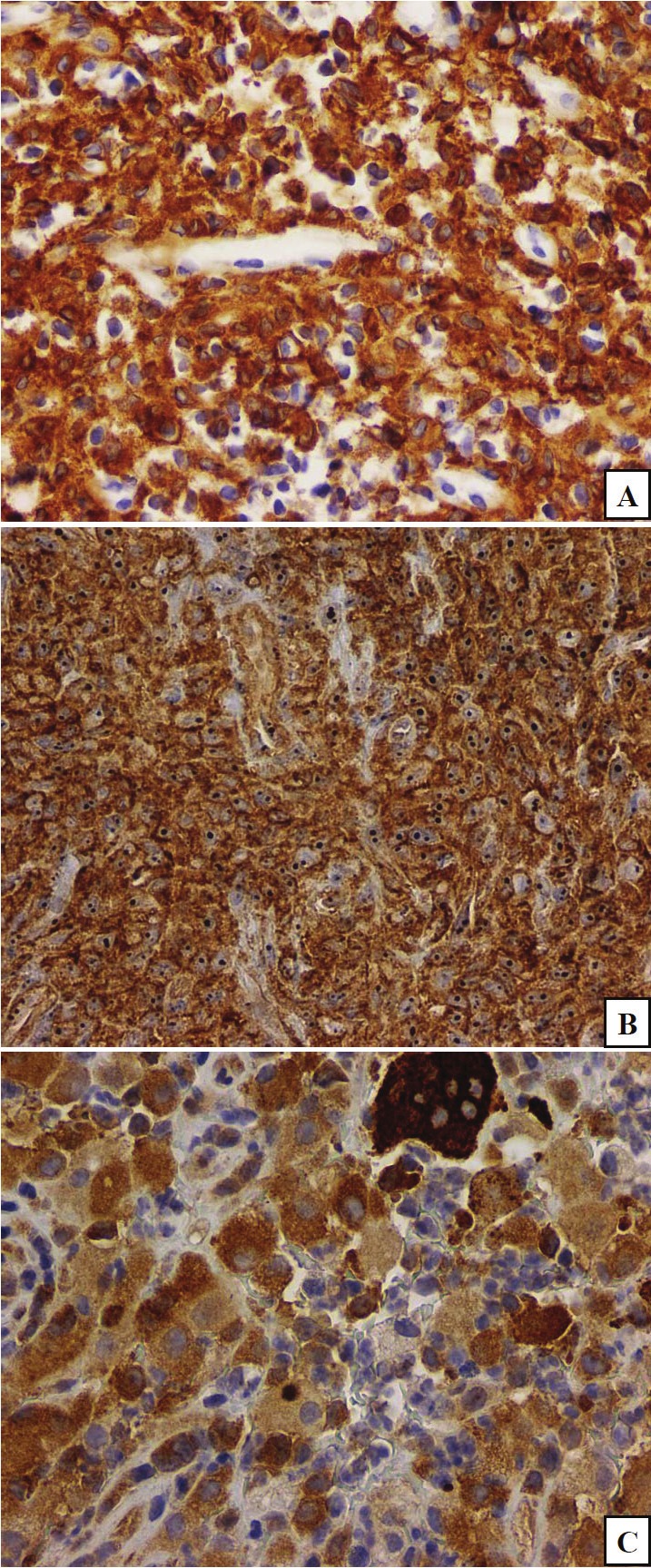
PKC-alpha (frame A) and phospholipase D1(PLD1;frame B) share a similar subcellular distribution on the plasmalemmal and in the cytoplasmic compartments of the majority of lesional cells. Fatty acid synthase (FASN;frame C) has variable intensity of cytoplasmic expression in the majority of the lesional cells. (original magnifications, x200 for A and B, x400 for C).
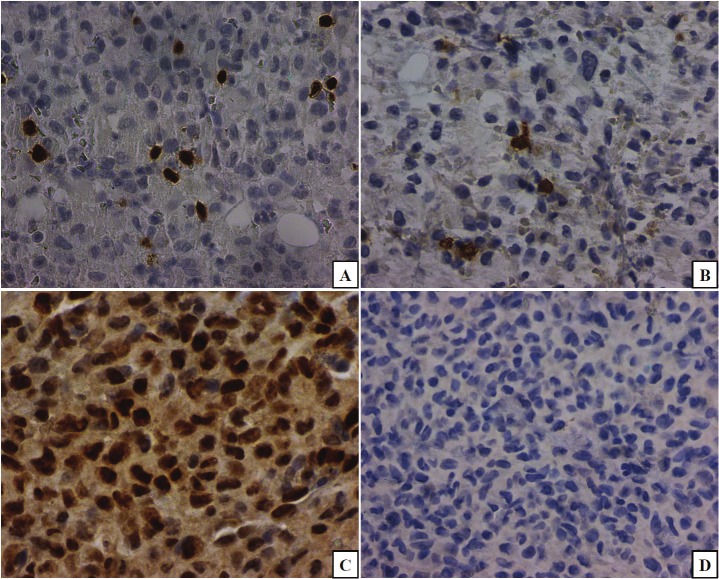
In an attempt to investigate a possible immune-dysregulation in Langerhans histiocytosis, we counted the FoxP3+ (Figure 3A) and CD8+ (Figure 3B) cells in ten high power fields, and their ratio was increased in these cases, being the highest (7.94:1) in case 5, in which there were polyostotic skull lesions and skin involvement with a poor response to treatment. The lowest FoxP3/CD8 ratio observed was 1.7:1. Correspondingly, p-STAT3 (Tyr 705) was expressed focally in the nuclei of lesional Langerhans cells but it was not observed in the multinucleated giant cells (Figure 3C). A negative immunohistochemical control is included in Figure 3D.
Figure 3.
The calculated ratio of FoxP3+/CD8+ lymphocytes, revealed an increased number of FoxP3+ cells (frame A) compared to CD8+ cells (frame B). The highest ratio corresponded to the case with the most aggressive clinical course (case 5). Relatedly, phosphorylated (p)-signal transducer and activator of transcription (STAT)3 (Tyr 705) is variably expressed in nuclei of the lesional Langerhans cells (frame C). A negative control is depicted in frame D. (original magnifications, x400 A-C and x200, D).
Discussion
Morphoproteomics utilizes morphology to determine the subcellular compartmentalization of both phosphorylated and non-phosphorylated protein analytes in evaluating their state of activation. Furthermore, by combining such findings with both the correlative expression of other proteins and the nature of companionate cells in the lesional microenvironment, morphoproteomics can provide clues to histogenesis, lesional biology, and potential therapeutic options [23,24].
Our morphoproteomic findings in the lesional cells of LCH to include nuclear Gli2 expression, plasmalemmal and cytoplasmic PKC- α and PLD1, and cytoplasmic FASN coincide with previous observations of TGF-β1 expression [20,25] and pathway signaling in LCH. Specifically, the effects of TGF-β1 signaling include osteoclastogenesis through IL-11 and Gli2-associated parathyroid hormone-related protein production [21,22,26-31]. Furthermore, the expression of IL-11 was previously demonstrated in LCH by Brown RE [20] and Andersson BU et al. [32], and its role in osteoclastogenesis was reviewed by Guk KD and co-workers [33], with the comment that this role was very well defined in vitro. Also, IL-11 signals through JAK/STAT pathway [34], and together with TGF-β1 pathway signaling [35] stimulate the genesis of FoxP3+ lymphocytes [36-40], contributing to the immune dysregulation of LCH. Correspondingly, we found an increased FoxP3+ to CD8+ ratio of lymphocytes in the five cases of LCH that we studied, and the ratio appeared to coincide with the aggressiveness of the disease. (The only case with extensive involvement of the cranium, with extension to the skin, had a ratio of FoxP3+/CD8+ lymphocytes of 7.94:1.) Such evidence of immune dysregulation was previously reported by Senechal et al. [7], who described a consistent expansion of T regulatory cells in biopsies from 40 patients with LCH. Furthermore, it accords with the gene expression profile in LCH lesion CD3+ cells of an activated regulatory T cell phenotype with increased expression of FOXP3 gene [41]. In addition, our finding of the expression of FASN could reflect TGF-β signaling given the latter’s apparent role in inducing FASN [42,43]. Moreover, it provides a correlate with the original observation of preferential lipid metabolism in histiocytosis X [44,45]. The expression and role of fatty acid synthase (FASN) has not been reported in LCH to date, as per a review of the National Library of Medicine’s Medline Data Base. We noticed variable expression of FASN in all cases, and this finding has possible pathogenetic implications with regard to PKC-α/PLD1/TGF-β signaling, as well as therapeutic implications (see schematic and legend for Figure 4; vide infra). As previously stated, the role of Gli2 in osteoclastogenesis is well known [26], and its expression in lesional Langerhans cells provides a correlate for TGF-β signaling via the non-canonical TGF-beta {Smad3}Gli2 signaling pathway in the genesis of multinucleated osteoclast-like cells in LCH [26-31]. Again, based on a review of the National Library of Medicine’s Medline Data Base, our study appears to be the first to demonstrate the nuclear expression of Gli2 in osteolytic LCH. The central role of TGF-β pathway signaling in incorporating these and previous observations in the osteoclastogenesis and immune dysregulation of osteolytic LCH is represented in the schematic in Figure 4 and detailed in the corresponding legend.
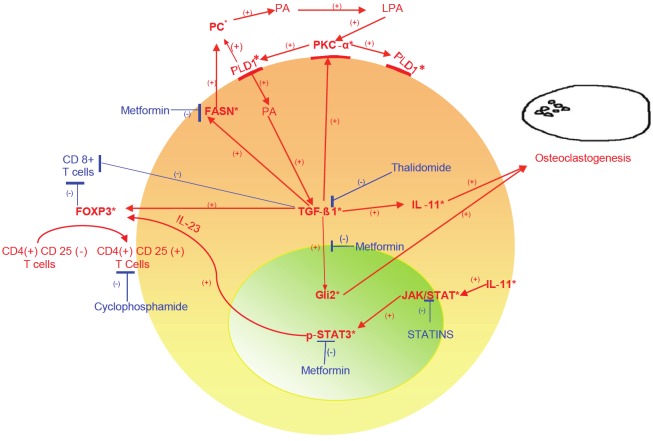
Figure 4.
Schematic depicting a central role for transforming growth factor (TGF)-β1 signaling in osteoclastogenesis and immune dysregulation of osteolytic Langerhans cell histiocytosis (LCH). Specifically, TGF-β1 expression [20,25] and signaling in osteolytic LCH correlates with and could lead to:1) interleukin (IL)-11 [20,22,28,32] and in turn, both osteoclastogenesis [21,33] and the activation of signal transducer and activator of transcription (STAT)3 pathway [34], evidenced by nuclear expression of phosphorylated (p)-STAT3 (Tyr 705) and subsequent expansion of the FoxP3 + population via IL-23 [36,37,41]; 2) nuclear expression of glioma-associated oncogene protein (Gli)2 [26,30] leading to the formation of parathyroid hormone-related protein (PTHrP) and then to activation of RANKL with osteoclastogenesis [26,31,46,47]; 3)fatty acid synthase (FASN) expression with lipogenesis to include phosphocholine (choline-phospholipids have been described in LCH [44]) and phosphatidlycholine [45], the substrate for phospholipase (PL)D1 with subsequent phosphatidic acid(PA) and lysophosphatidic acid (LPA) formation and in turn to complex activation of protein kinase C (PKC)-α, PLD1 and TGF-β1 [48-57]; 4) activation of PKC-α [58-60]; and 5) downregulation of CD8+ cell-associated immune surveillance[61] and with activation of T regulatory (FoxP3+) cells [35,38-40]. Targeted therapeutic possibilities in this context include metformin to : 1. Activate AMPK with inhibition of TGF-β{Smad3}-associated Gli2 and IL-11 signaling [26-31,62,63] and in turn, inhibition of PTHrP and RANKL signaling [46,64,65] with reduced osteoclastogenesis; 2. Downregulate FASN [66] leading to reduced substrate for the PLD1/PKC-α/TGF-β signaling axis; and 3. Inhibit STAT3 activation at tyrosine 705 [67]. Moderators of TGF-β pathway signaling related to immune dysregulation include: thalidomide, which has shown efficacy in blocking the TGF-β pathway [16-19] and clinically, in treating osteolytic LCH [15]; and metronomic cyclophosphamide , which targets T regulatory cells [68]. *Indicates protein analytes identified in osteolytic LCH.
Finally, the combination of morphoproteomic and morphometric findings in the study, although based on a small series of cases, raise possible therapeutic options that would combine to inhibit TGF-beta pathway signaling and immune dysregulation, the latter by targeting T regulatory (FoxP3+) cells. This is also summarized in the schematic (Figure 4 and legend).
In conclusion, this study reinforces the importance of an activated TGF-β pathway in the biology and histopathology of osteolytic LCH, with the immediate effects to include osteoclastogeneis and expansion of T regulatory cells. It also raises possible therapeutic strategies to target this central TGF-β signaling in the treatment of osteolytic LCH.
Aknowledgements
We thank Pamela K. Johnston, HT (ASCP) for technical support and Ms. Bheravi Patel for secretarial support and assistance with the graphics.
References
- 1.Degar BA, Rollins BJ. Langerhans cell histiocytosis: malignancy or inflammatory disorder doing a great job of imitating one? Disease Models and Mechanisms. 2009;2:436–439. doi: 10.1242/dmm.004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geissmann F, Lepelletier Y, Fraitag S, Valladeau J, Bodemer C, Debré M, Leborgne M, Saeland S, Brousse N. Differentiation of Langerhans cells in Langerhans cell histiocytosis. Blood. 2001;97:1241–1248. doi: 10.1182/blood.v97.5.1241. [DOI] [PubMed] [Google Scholar]
- 3.Aricò M, Nichols K, Whitlock JA, Arceci R, Haupt R, Mittler U, Kühne T, Lombardi A, Ishii E, Egeler RM, Danesino C. Familial clustering of Langerhans cell histiocytosis. Br J Haematol. 1999;107:883–888. doi: 10.1046/j.1365-2141.1999.01777.x. [DOI] [PubMed] [Google Scholar]
- 4.Da Costa CE, Szhuai K, van Eijk R, Hoogeboom M, Sciot R, Mertens F. No genomic aberrations in Langerhans cell histiocytosis as assessed by diverse molecular technologies. Genes Chromosomes Cancer. 2009;48:239–249. doi: 10.1002/gcc.20634. [DOI] [PubMed] [Google Scholar]
- 5.Badalian-Very G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, Calicchio ML, Kuo FC, Ligon AH, Stevenson KE, Kehoe SM, Garraway LA, Hahn WC, Meyerson M, Fleming MD, Rollins BJ. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919–23. doi: 10.1182/blood-2010-04-279083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleming MD, Pinkus JL, Fournier MV, Alexander SW, Tam C, Loda M, Sallan SE, Nichols KE, Carpentieri DF, Pinkus GS, Rollins BJ. Coincident expression of the chemokine receptors CCR6 and CCR7 by pathologic Langerhans cells in Langerhans cell histiocytosis. Blood. 2003;101:2474–2475. doi: 10.1182/blood.V101.7.2473. [DOI] [PubMed] [Google Scholar]
- 7.Senechal B, Elain G, Jeziorski E, Grondin V, Patey-Mariaud de Serre N, Jaubert F, Beldjord K, Lellouch A, Glorion C, Zerah M, Mary P, Barkaoui M, Emile JF, Boccon-Gibod L, Josset P, Debré M, Fischer A, Donadieu J, Geissmann F. Expansion of regulatory T cells in patients with Langerhans cell histiocytosis. PLoS Med. 2007;4:e253. doi: 10.1371/journal.pmed.0040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Farran RP, Zaretski E, Egeler RM. Treatment of Langerhans cell histiocytosis with pamidronate. J Pediatr Hematol Oncol. 2001;23:54–56. doi: 10.1097/00043426-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Brown RE. More on pamidronate in Langerhans’-cell histiocytosis. N Engl J Med. 2001;345:1503. [PubMed] [Google Scholar]
- 11.Kamizono J, Okada Y, Shirahata A, Tanaka Y. Bisphosphonate induces remission of refractory osteolysis in langerhans cell histiocytosis. J Bone Miner Res. 2002;17:1926–1928. doi: 10.1359/jbmr.2002.17.11.1926. [DOI] [PubMed] [Google Scholar]
- 12.Sivendran S, Harvey H, Lipton A, Drabick J. Treatment of Langerhans cell histiocytosis bone lesions with zoledronic acid: a case series. Int J Hematol. 2011;93:782–786. doi: 10.1007/s12185-011-0839-2. [DOI] [PubMed] [Google Scholar]
- 13.Tsuda H, Yamasaki H, Tsuji T. Resolution of bone lysis in Langerhans cell histiocytosis by bisphosphonate therapy. Br J Haematol. 2011;154:287. doi: 10.1111/j.1365-2141.2011.08654.x. [DOI] [PubMed] [Google Scholar]
- 14.Morimoto A, Shioda Y, Imamura T, Kanegane H, Sato T, Kudo K, Nakagawa S, Nakadate H, Tauchi H, Hama A, Yasui M, Nagatoshi Y, Kinoshita A, Miyaji R, Anan T, Yabe M, Kamizono J LCH Committee, Japanese Pediatric Leukemia/Lymphoma Study Group. Nationwide survey of bisphosphonate therapy for children with reactivated Langerhans cell histiocytosis in Japan. Pediatr Blood Cancer. 2011;56:110–115. doi: 10.1002/pbc.22703. [DOI] [PubMed] [Google Scholar]
- 15.McClain KL, Kozinetz CA. A phase II trial using thalidomide for Langerhans cell histiocytosis. Pediatr Blood Cancer. 2007;48:44–49. doi: 10.1002/pbc.20578. [DOI] [PubMed] [Google Scholar]
- 16.Choe JY, Jung HJ, Park KY, Kum YS, Song GG, Hyun DS, Park SH, Kim SK. Anti-fibrotic effect of thalidomide through inhibiting TGF-beta-induced ERK 1/2 pathways in bleomycin-induced lung fibrosis in mice. Inflamm Res. 2010;59:177–188. doi: 10.1007/s00011-009-0084-9. [DOI] [PubMed] [Google Scholar]
- 17.Arai H, Furusu A, Nishino T, Obata Y, Nakazawa Y, Nakazawa M, Hirose M, Abe K, Koji T, Kohno S. Thalidomide prevents the progression of peritoneal fibrosis in mice. Acta Histochem Cytochem. 2011;44:51–60. doi: 10.1267/ahc.10030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giannopoulos K, Schmitt M, Własiuk P, Chen J, Bojarska-Junak A, Kowal M, Roliñski J, Dmoszyñska A. The high frequency of T regulatory cells in patients with B-cell chronic lymphocytic leukemia is diminished through treatment with thalidomide. Leukemia. 2008;22:222–224. doi: 10.1038/sj.leu.2404869. [DOI] [PubMed] [Google Scholar]
- 19.Giannopoulos K, Dmoszynska A, Kowal M, Wasik-Szczepanek E, Bojarska-Junak A, Rolinski J, Döhner H, Stilgenbauer S, Bullinger L. Thalidomide exerts distinct molecular antileukemic effects and combined thalidomide/fludarabine therapy is clinically effective in high-risk chronic lymphocytic leukemia. Leukemia. 2009;23:1771–1778. doi: 10.1038/leu.2009.98. [DOI] [PubMed] [Google Scholar]
- 20.Brown RE. Angiotensin-converting enzyme, transforming growth factor beta (1), and inteleukin 11 in the osteolytic lesions of Langerhans cell histiocytosis. Arch Pathol Lab Med. 2000;124:1287–1290. doi: 10.5858/2000-124-1287-ACETGF. [DOI] [PubMed] [Google Scholar]
- 21.Kudo O, Sabokbar A, Popcock A, Itonaga I, Fujikawa Y, Athanasou NA. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003;32:1–7. doi: 10.1016/s8756-3282(02)00915-8. [DOI] [PubMed] [Google Scholar]
- 22.Maier R, Ganu V, Lotz M. Interleukin-11, an inducible cytokine in human articular chondrocytes and synoviocytes, stimulates the production of the tissue inhibitor of metalloproteinases. J Biol Chem. 1993;268:21527–21532. [PubMed] [Google Scholar]
- 23.Brown RE. Morphoproteomics: exposing protein circuitries in tumors to identify potential therapeutic targets in cancer patients. Expert Rev Proteomics. 2005;2:337–348. doi: 10.1586/14789450.2.3.337. [DOI] [PubMed] [Google Scholar]
- 24.Brown RE. Morphogenomics and morphoproteomics: A role for Anatomic Pathology in Personalized Medicine. Arch Pathol Lab Med. 2009;133:568–579. doi: 10.5858/133.4.568. [DOI] [PubMed] [Google Scholar]
- 25.de Graaf JH, Tamminga RY, Dam-Meiring A, Kamps WA, Timens W. The presence of cytokines in Langerhans’ cell histiocytosis. J Pathol. 1996;180:400–406. doi: 10.1002/(SICI)1096-9896(199612)180:4<400::AID-PATH701>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 26.Johnson RW, Nguyen MP, Padalecki SS, Grubbs BG, Merkel AR, Oyajobi BO, Matrisian LM, Mundy GR, Sterling JA. TGF-beta promotion of Gli2-induced expression of parathyroid hormonerelated protein, an important osteolytic factor in bone metastasis, is independent of canonical Hedgehog signaling. Cancer Res. 2011;71:822–831. doi: 10.1158/0008-5472.CAN-10-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiuru M, Solomon J, Ghali B, van der Meuten M, Crystal RG, Hidaka C. Transient overexpression of sonic hedgehog alters the architecture and mechanical properties of trabecular bone. J Bone Miner Res. 2009;24:1598–1607. doi: 10.1359/jbmr.090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta J, Robbins J, Jilling T, Seth P. TGFbeta-dependent induction of interleukin 11 and interleukin-8 involves SMAD and p38 MAPK pathways in breast tumor models with varied bone metastases potential. Cancer Biol Ther. 2011;11:311–316. doi: 10.4161/cbt.11.3.14096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guise TA, Chirgwin JM. Transforming growth factor-beta in osteolytic breast cancer bone metastases. Clin Orthop Relat Res. 2003:S32–38. doi: 10.1097/01.blo.0000093055.96273.69. [DOI] [PubMed] [Google Scholar]
- 30.Dennler S, André J, Alexaki I, Li A, Magnaldo T, ten Dijke P, Wang XJ, Verrecchia F, Mauviel A. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6986. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- 31.Nakchbandi IA, Weir EE, Insogna KL, Philbrick WM, Broadus AE. Parathyroid hormone-related protein induces spontaneous osteoclast formation via a paracrine cascade. Proc Natl Acad Sci USA. 2000;97:7296–7300. doi: 10.1073/pnas.110553397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersson BU, Tani E, Andersson U, Henter JI. Tumor necrosis factor, interleukin 11, and leukemia inhibitory factor produced by Langerhans cells in Langerhans cell histiocytosis. J Pediatr Hematoll Oncol. 2004;26:706–711. doi: 10.1097/00043426-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Guk KD, Kuprash DV. Interleukin-11 and IL-6 like cytokine. Mol Biol. 2011;45:44–45. [PubMed] [Google Scholar]
- 34.Wang RJ, Peng RY, Gao YB, Chang GM, Xu XP, Fu KF. Jak/STAT signaling pathway of IL-11 in the protection of intestinal epithelial cells from neuron radiation. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2009;25:27–30. [PubMed] [Google Scholar]
- 35.Shan vitro human TGF-beta treatment converts CD4(+)CD25(-) T cells into induced T regulatory like cells. Vet Immunoll Immunopathol. 2010;137:161–165. doi: 10.1016/j.vetimm.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 36.Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langrish CL, McKenzie BS, Wilson JF, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immuno Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 38.Baratelli F, Lee JM, Hazra S, Lin Y, Walser TC, Schaue D, Pak PS, Elashoff D, Reekamp K, Zhang C, Fishbein MC, Sharma S, Dubinett SM. PGE(2) contributes to TGF-beta induced T regulatory cell function in human non-small cell lung cancer. Am J Transl Res. 2010;2:356–367. [PMC free article] [PubMed] [Google Scholar]
- 39.Lu L, Ma J, Wang X, Wang J, Zhang F, Yu J, He G, Xu B, Brand DD, Horwitz DA, Shi W, Zheng SG. Synergistic effect of TGF-beta superfamily members on the induction of FoxP3+Treg. Eur J Immunol. 2010;40:142–152. doi: 10.1002/eji.200939618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pyzik M, Piccirllo CA. TGF-beta1 modulates FoxP3 expression and regulatory activity in distinct CD4+ T cell subsets. J Leukooc Biol. 2007;82:335–346. doi: 10.1189/jlb.1006644. [DOI] [PubMed] [Google Scholar]
- 41.Allen CE, Li L, Peters TL, Leung HC, Yu A, Man TK, Gurusiddappa S, Phillips MT, Hicks MJ, Gaikwad A, Merad M, McClain KL. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184:4557–4567. doi: 10.4049/jimmunol.0902336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teruel T, Valverde AM, Benito M, Lorenzo M. Transforming growth factor beta 1 induces differentiation-specific gene expression in fetal rat brown adipocytes. FEBS Lett. 1995;364:193–197. doi: 10.1016/0014-5793(95)00385-m. [DOI] [PubMed] [Google Scholar]
- 43.Saito K, Ishizaka N, Hara M, Matsuzaki G, Sata M, Mori I, Ohno M, Nagai R. Lipid accumulation and transforming growth factor-beta upregulation in the kidneys of rats administered angiotensin II. Hypertension. 2005;46:1180–1185. doi: 10.1161/01.HYP.0000184653.75036.d5. [DOI] [PubMed] [Google Scholar]
- 44.Barbey S, Despres S, Nezelof C. Histiocytosis X. Histochemical study of intracytoplasmic lipids. Apropos of 3 cases. Pathol Biol(Paris) 1975;23:639–646. [PubMed] [Google Scholar]
- 45.Ross J, Najjar AM, Sankaranarayanapillai M, Tong WP, Kaluarachchi K, Ronen SM. Fatty acid synthase inhibition results in a magnetic resonance-detectable drop in phosphocholine. Mol Cancer Ther. 2008;7:2556–2565. doi: 10.1158/1535-7163.MCT-08-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishii R, Morimoto A, Ikushima S, Sugimoto T, Asami K, Bessho F, Kudo K, Tsunematu Y, Fujimoto J, Imashuku S. High serum values of soluble CD154, IL-2 receptor, RANKL and osteoprotegerin in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2006;47:194–199. doi: 10.1002/pbc.20595. [DOI] [PubMed] [Google Scholar]
- 47.Cowan RW, Singh G, Ghert M. PTHrP increases RANKL expression by stromal cells from giant cell tumor of bone. J Orthop Res. 2011 doi: 10.1002/jor.22020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Hu T, Exton JH. Mechanisms of regulation of phospholipase D1 by protein kinase Calpha. J Biol Chem. 2003;278:2348–2355. doi: 10.1074/jbc.M210093200. [DOI] [PubMed] [Google Scholar]
- 49.Jenkins GM, Frohman MA. Phospholipase D: a lipid centric review. Cell Mol Life Sci. 2005;62:2305–2316. doi: 10.1007/s00018-005-5195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melendez AJ, Allen JM. Phospholipase D and immune receptor signalling. Semin Immunol. 2002;14:49–55. doi: 10.1006/smim.2001.0341. [DOI] [PubMed] [Google Scholar]
- 51.Piazza GA, Ritter JL, Baracka CA. Lysophosphatidic acid induction of transforming growth factors alpha and beta: modulation of proliferation and differentiation in cultured human keratinocytes and mouse skin. Exp Cell Res. 1995;216:51–64. doi: 10.1006/excr.1995.1007. [DOI] [PubMed] [Google Scholar]
- 52.Zhou BH, Chen JS, Chai MQ, Zhao S, Liang J, Chen HH, Song JG. Activation of phospholipase D activity in transforming growth factor-beta-induced cell growth inhibition. Cell Res. 2000;10:139–149. doi: 10.1038/sj.cr.7290043. [DOI] [PubMed] [Google Scholar]
- 53.van der Bend RL, de Widt J, van Corven EJ, Moolenaar WH, van Blitterswijk WJ. The biologically active phospholipid, lysophosphatidic acid, induces phosphatidylcholine breakdown in fibroblasts via activation of phospholipase D. Comparison with the response to endothelin. Biochem J. 1992;285:235–240. doi: 10.1042/bj2850235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong JH, Oh SO, Lee M, Kim YR, Kim DU, Hur GM, Lee JH, Lim K, Hwang BD, Park SK. Enhancement of lysophosphatidic acid-induced ERK phosphorylation by phospholipase D1 via the formation of phosphatidic acid. Biochem Biophys Res Commun. 2001;281:1337–1342. doi: 10.1006/bbrc.2001.4517. [DOI] [PubMed] [Google Scholar]
- 55.Jeon ES, Kim JH, Ryu H, Kim EK. Lysophosphatidic acid activates TGFBIp expression in human corneal fibroblasts through a TGF-beta1-dependent pathway. Cell Signal. 2012;24:1241–50. doi: 10.1016/j.cellsig.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 56.Jeon ES, Moon HJ, Lee MJ, Song HY, Kim YM, Cho M, Suh DS, Yoon MS, Chang CL, Jung JS, Kim JH. Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblast-like cells. Stem Cells. 2008;26:789–797. doi: 10.1634/stemcells.2007-0742. [DOI] [PubMed] [Google Scholar]
- 57.Lindschau C, Quass P, Menne J, Güler F, Fiebeler A, Leitges M, Luft FC, Haller H. Glucoseinduced TGF-beta1 and TGF-beta receptor-1 expression in vascular smooth muscle cells is mediated by protein kinase C-alpha. Hypertension. 2003;42:335–341. doi: 10.1161/01.HYP.0000087839.72582.DD. [DOI] [PubMed] [Google Scholar]
- 58.Zhou HY, Chen WD, Zhu DL, Wu LY, Zhang J, Han WQ, Li JD, Yan C, Gao PJ. The PDE1A-PK-Calpha signaling pathway is involved in the upregulation of alpha-smooth muscle actin by TGF-beta1 in adventitial fibroblasts. J Vasc Res. 2010;47:9–15. doi: 10.1159/000231716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chow JY, Dong H, Quach KT, Van Nguyen PN, Chen K, Carethers JM. TGF-beta mediates PTEN suppression and cell motility through calcium-dependent PKC-alpha activation in pancreatic cancer cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G899–905. doi: 10.1152/ajpgi.00411.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chakrabarty S, Huang S. Role of protein kinase C alpha in the induction of carcinoembryonic antigen by transforming growth factor beta 1. J Cell Physiol. 1995;164:148–153. doi: 10.1002/jcp.1041640119. [DOI] [PubMed] [Google Scholar]
- 61.Kim YJ, Stringfield TM, Chen Y, Broxmeyer HE. Modulation of cord blood CD8+ T-cell effector differentiation by TGF-beta1 and 4-1BB costimulation. Blood. 2005;105:274–281. doi: 10.1182/blood-2003-12-4343. [DOI] [PubMed] [Google Scholar]
- 62.Xiao H, Ma X, Feng W, Fu Y, Lu Z, Xu M, Shen Q, Zhu Y, Zhang Y. Metformin attenuates cardiac fibrosis by inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc Res. 2010;87:504–513. doi: 10.1093/cvr/cvq066. [DOI] [PubMed] [Google Scholar]
- 63.Brown RE, Weerasinghe P. Metformin activates AMP kinase and inhibits Gli2 expression in a human neuroblastoma cell line. Unpublished observation. [Google Scholar]
- 64.Lee YS, Kim YS, Lee SY, Kim GH, Kim BJ, Lee SH, Lee KU, Kim GS, Kim SW, Koh JM. AMP kinase acts as a negative regulator of RANKL in the differentiation of osteoclasts. Bone. 2010;47:926–937. doi: 10.1016/j.bone.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 65.Mai QG, Zhang ZM, Xu S, Lu M, Zhou RP, Zhao L, Jia CH, Wen ZH, Jin DD, Bai XC. Metformin stimulates osteoprotegerin and reduces RANKL expression in osteoblasts and ovariectomized rats. J Cell Biochem. 2011;112:2902–2909. doi: 10.1002/jcb.23206. [DOI] [PubMed] [Google Scholar]
- 66.Algire C, Amrein L, Zakikhani M, Panasci L, Pollak M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr Relat Cancer. 2010;17:351–360. doi: 10.1677/ERC-09-0252. [DOI] [PubMed] [Google Scholar]
- 67.Deng XS, Wang S, Deng A, Liu B, Edgerton SM, Lind SE, Wahdan-Alaswad R, Thor AD. Metformin targets Stat3 to inhibit cell growth and induce apoptosis in triple-negative breast cancers. Cell Cycle. 2012;11:367–376. doi: 10.4161/cc.11.2.18813. [DOI] [PubMed] [Google Scholar]
- 68.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]