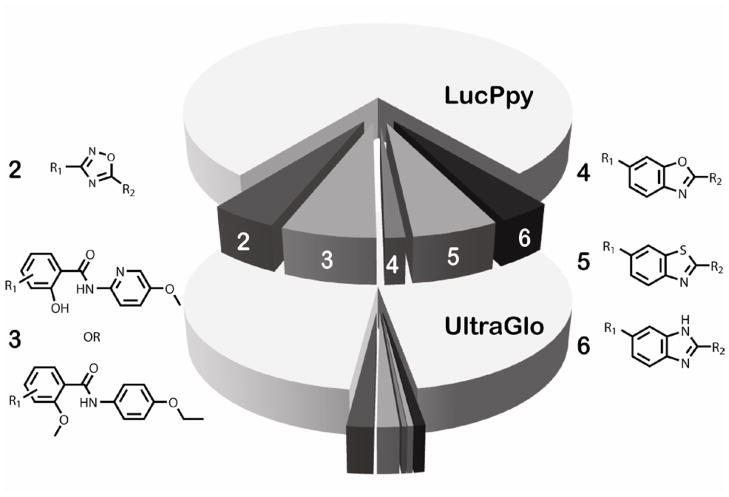
Figure 3. Comparison of inhibitor scaffold representation between lucPpy and Ultra-Glo luciferase formulations.
Center pie charts represent the amount of active (darker grey slices) and related inactive analogs (light grey area in each pie chart) for prominent luciferase inhibitor scaffolds (2–6) for the lucPpy (top pie) and Ultra-Glo luciferase (bottom pie) qHTS. The scaffolds and the associated analogs were determined as described in Auld et al.15 The number of lucPpy active analogs was reduced from 70 to 27 (2, 3,5-diaryl-oxadiazoles), 98 to 23 (3, benzamides), 21 to 5 (4, benzoxazoles), 89 to 6 (5, benzthiazoles), 55 to 11 (6, benzimidazoles) in the Ultra-Glo luciferase qHTS. The corresponding number of structurally related inactive analogs increased from 1,192 to 1,442 in the Ultra-Glo luciferase qHTS.