Table 3.
Characterization of selected 2-phenylbenzothiazole analogs
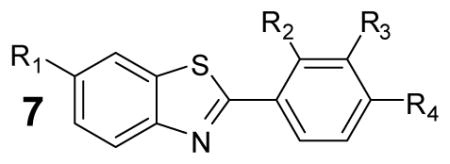
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Analogue # | R1 | R2 | R3 | R4 | IC50Ultr aGlo | IC50lucPpy | IC50KinGlo | IC50KinGlo Plus | IC50KinGlo Max |
| a | H | H | H | dimethylamine | 0.32± 0.0 | 0.2±0.0 | 10.7±3.4 | Inactive | Inactive |
| b | H | H | H | Cl | 1.4± 0.18 | 4.5±0.0 | >50 | Inactive | Inactive |
| c | OMe | H | H | F | 0.86± 0.08 | 1.3±0.09 | >50 | Inactive | Inactive |
| d | OMe | H | F | H | 1.1± 0.14 | 1.5±0.1 | >50 | Inactive | Inactive |
| e | OMe | F | H | H | 0.5± 0.05 | 0.6±0.0 | >50 | Inactive | Inactive |
| f | OMe | Cl | H | F | 4.9± 1.1 | 5.4±0.7 | >50 | Inactive | Inactive |
| g | OMe | H | H | Cl | 3.8± 0.5 | 3.5±0.0 | >50 | Inactive | Inactive |
| h | OMe | O-methylbenzene | H | H | 4.3± 0.2 | 8.9±0.6 | >50 | Inactive | Inactive |
| i | OMe | H | O-methylbenzene | H | 30± 3 | 13.4±1 | Inactive | Inactive | Inactive |
| j | OMe | H | H | CN | 6.1± 1.3 | 5.7±0.9 | Inactive | Inactive | Inactive |
| k | OMe | H | H | OMe | 4.1± 0.4 | 2.2±0.0 | >50 | Inactive | Inactive |
| l | OMe | OMe | H | H | 5.6± 1.3 | 3.2±0.0 | >50 | Inactive | Inactive |
| m | OMe | H | OMe | OMe | 3.8± 0.6 | 2.8±0.0 | >50 | Inactive | Inactive |
| n | OMe | H | acetamide | H | 1.9± 0.4 | 2.9±0.2 | >50 | Inactive | Inactive |
| o | OMe | H | Me | H | 0.67± 0.07 | 1.5±0.1 | >50 | Inactive | Inactive |
| p | OMe | H | H | dimethylamine | 2.7± 0.6 | 0.7±0.04 | >50 | Inactive | Inactive |
| q | H | H | F | H | 7.8± 0.3 | 24.2±1.6 | Inactive | Inactive | Inactive |
| r | H | H | OMe | OMe | 1.9± 0.3 | 3.2±0.0 | >50 | Inactive | Inactive |
| s | H | H | H | OMe | 0.36± 0.02 | 1.0±0.0 | >50 | Inactive | Inactive |
| t | H | OMe | H | H | 7.7± 1.4 | 11.3±1.8 | >50 | Inactive | Inactive |
| u | H | H | H | CN | 2.1± 0.2 | 6.3±0.0 | >50 | Inactive | Inactive |
| v | H | H | H | acetamide | 3.1± 0.5 | 3.0±0.2 | >50 | Inactive | Inactive |
Activity of compounds was determined by measurement of luminescence using either purified luciferase enzyme assays (IC50UltraGlo or IC50lucPpy) or formulations of UltraGlo (IC50KinGlo , IC50K inGlo Plus , IC50KinGlo Max). Data shown are mean± SD for at least three replications.
