Fig 4.
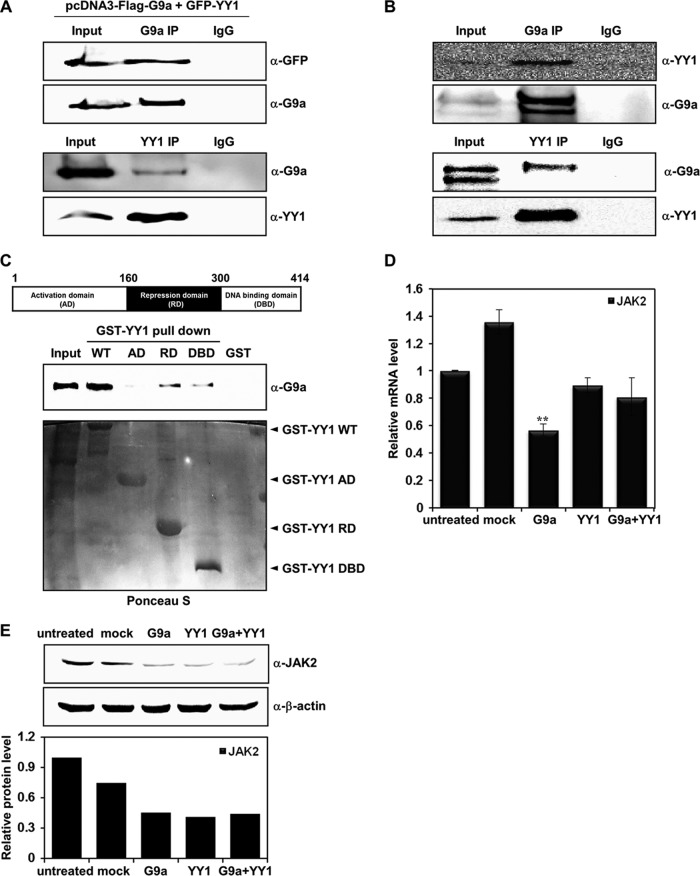
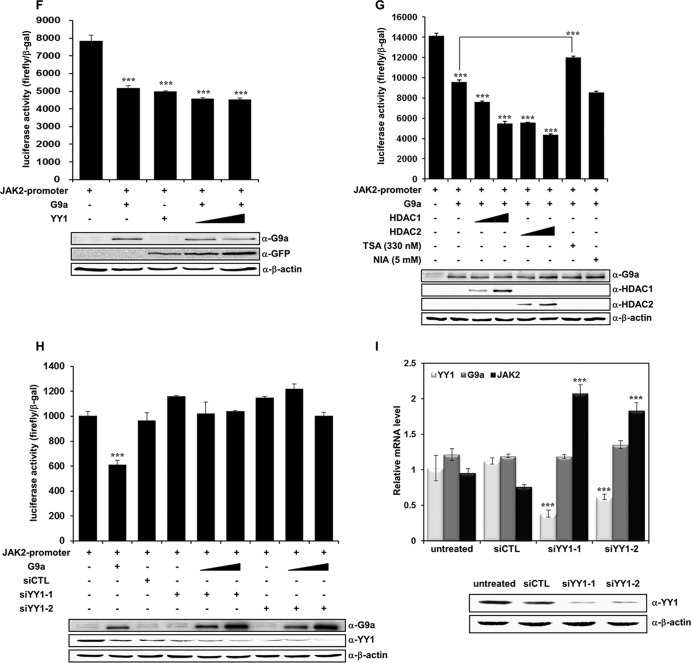
YY1 is involved in G9a-mediated JAK2 downregulation. (A) HEK293T cells were cotransfected with pcDNA3-Flag-G9a and pEGFP-YY1 constructs. Anti-G9a and anti-YY1 immunoprecipitates were analyzed by Western blot analysis with anti-GFP and anti-G9a antibodies. (B) G9a endogenously interacts with YY1 in K562 cells. Anti-G9a and anti-YY1 immunoprecipitates from K562 whole-cell extracts were analyzed by Western blot analysis with anti-YY1 and anti-G9a antibodies. (C) Cell lysates from G9a-overexpressed HEK293T cells were incubated with GST, the GST-YY1 wild type, or GST-YY1 deletion mutants and immunoblotted with anti-G9a antibody. The activation domain (AD), repression domain (RD), and DNA binding domain (DBD) of YY1 are shown. The levels of full-length YY1 and YY1 deletion mutants were determined by Ponceau S staining. (D) JAK2 mRNA levels were analyzed in transfected K562 cells by real-time PCR. K562 cells were transfected with pcDNA3, pcDNA3-Flag-G9a, and pEGFP-YY1 and cotransfected with pcDNA3-Flag-G9a and pEGFP-YY1. Results are shown as means ± SD (n = 3). **, P < 0.01 compared with the value for the untreated control. (E) K562 cells were transfected with the indicated DNA constructs, and JAK2 protein levels were confirmed via Western blot analysis. β-Actin was used as a loading control. Results of the Western blot were quantified and normalized to the level of β-actin. (F) K562 cells were transfected with the pGL4.12-JAK2 promoter (1 μg), pcDNA3-Flag-G9a (1 μg), and pEGFP-YY1 (1 and 1.5 μg). Following transfection, cells were grown for 48 h, and cell extracts were assayed for luciferase activity. Expression of the transfected constructs are shown in the Western blots. (G) pGL4.12-JAK2 promoter (1 μg), pcDNA3-Flag-G9a (0.5 μg), pCMX-HDAC1 (0.5 and 1 μg), and pcDNA3.1-HDAC2 (0.5 and 1 μg) were transfected into K562 cells. Twenty-four hours after transfection, 330 nM trichostatin A (TSA) or 5 mM nicotinamide (NIA) were added for 24 h, and luciferase activities were measured. G9a, HDAC1, and HDAC2 expressions were confirmed by Western blot analysis. (H) K562 cells were transfected with the pGL4.12-JAK2 promoter (1 μg), pcDNA3-Flag-G9a (0.5 μg, 1 μg), si-CTL RNA (100 nM), and si-YY1 RNAs (100 nM), along with the β-galactosidase expression plasmid. Luciferase activity was measured 48 h after the transfection. G9a overexpression and YY1 knockdown by two different si-YY1 RNAs are shown in the Western blots. (F, G, and H) Luciferase activities were normalized to that of β-galactosidase, and the results are presented as means ± SD (n = 4). ***, P < 0.001 compared with the value for the reporter plasmid alone. (I) K562 cells were transfected with si-CTL RNA (100 nM) or si-YY1 RNAs (100 nM). YY1, G9a, and JAK2 expression levels were confirmed by real time-PCR. YY1 knockdown by si-YY1 RNAs is shown in the Western blots.