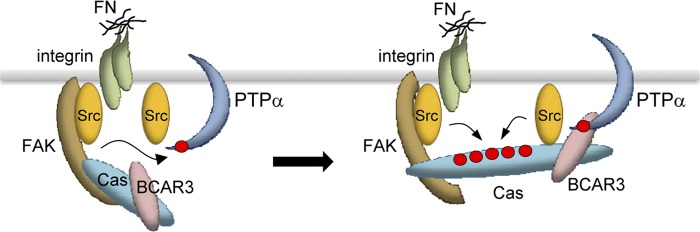
Fig 9.
Schematic diagram of the mechanism by which phospho-Tyr789 PTPα coordinates Cas positioning and phosphorylation in focal adhesions. Left, fibronectin (FN) engagement of integrins induces PTPα phosphorylation at Tyr789 by activated Src in the Src-FAK complex. Right, phospho-Tyr789 PTPα is localized to forming focal adhesions. BCAR3 forms a molecular bridge between phospho-PTPα and Cas (with the SH2 domain of BCAR3 binding to phosphoTyr789 on PTPα and the GEF domain of BCAR3 associating with the C-terminal region of Cas), thus positioning Cas in focal adhesions. The PTPα-BCAR3-dependent C-terminal anchoring of Cas promotes Cas interaction with Src and Src-mediated phosphorylation and activation of Cas. This may be facilitated by FAK-mediated anchoring of the N-terminal of Cas in focal adhesions so that dual opposing Cas anchors enable stretching and increased SD region accessibility to phosphorylation. Cas phosphorylation enables optimal signaling to Crk, Rac1, Cdc42, and PAK and cell migration (not shown).